NEET-XI-Chemistry
05: States of Matter
- #15Calculate the total pressure in a mixture of 8 g of dioxygen and 4 g of dihydrogen confined in a vessel of 1 dm3 at 27°C. R = 0.083 bar dm3 K-1 mol-1.
Ans : Given,
Mass of dioxygen (O2) = 8 g
Thus, number of moles of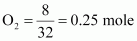
Mass of dihydrogen (H2) = 4 g
Thus, number of moles of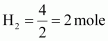
Therefore, total number of moles in the mixture = 0.25 + 2 = 2.25 mole
Given,
V = 1 dm3
n = 2.25 mol
R = 0.083 bar dm3 K-1 mol-1
T = 27°C = 300 K
Total pressure (p) can be calculated as:
pV = nRT
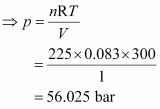
Hence, the total pressure of the mixture is 56.025 bar.