NEET-XII-Chemistry
07: The p-Block Elements
- Qstn #27Give two examples to show the anomalous behaviour of fluorine.
Ans : Anomalous behaviour of fluorine
(i) It forms only one oxoacid as compared to other halogens that form a number of oxoacids.
(ii) Ionisation enthalpy, electronegativity, and electrode potential of fluorine are much higher than expected.
- Qstn #28Sea is the greatest source of some halogens. Comment.
Ans : Sea water contains chlorides, bromides, and iodides of Na, K, Mg, and Ca. However, it primarily contains NaCl. The deposits of dried up sea beds contain sodium chloride and carnallite, KCl.MgCl2.6H2O. Marine life also contains iodine in their systems. For example, sea weeds contain upto 0.5% iodine as sodium iodide. Thus, sea is the greatest source of halogens.
- Qstn #29Give the reason for bleaching action of Cl2.
Ans : When chlorine reacts with water, it produces nascent oxygen. This nascent oxygen then combines with the coloured substances present in the organic matter to oxide them into colourless substances.

Coloured substances + [O] → Oxidized colourless substance
- Qstn #30Name two poisonous gases which can be prepared from chlorine gas.
Ans : Two poisonous gases that can be prepared from chlorine gas are
(i) Phosgene (COCl2)
(ii) Mustard gas (ClCH2CH2SCH2CH2Cl)
- Qstn #31Why is ICl more reactive than I2?
Ans : ICl is more reactive than I2 because I-Cl bond in ICl is weaker than I-I bond in I2.
- Qstn #32Why is helium used in diving apparatus?
Ans : Air contains a large amount of nitrogen and the solubility of gases in liquids increases with increase in pressure. When sea divers dive deep into the sea, large amount of nitrogen dissolves in their blood. When they come back to the surface, solubility of nitrogen decreases and it separates from the blood and forms small air bubbles. This leads to a dangerous medical condition called bends. Therefore, air in oxygen cylinders used for diving is diluted with helium gas. This is done as He is sparingly less soluble in blood.
- Qstn #33Balance the following equation: XeF6 + H2O → XeO2F2 + HF
Ans : Balanced equation
XeF6 + 2 H2O → XeO2F2 + 4 HF
- Qstn #34Why has it been difficult to study the chemistry of radon?
Ans : It is difficult to study the chemistry of radon because it is a radioactive substance having a half-life of only 3.82 days. Also, compounds of radon such as RnF2 have not been isolated. They have only been identified.
- #Section : IISECTION I Page No 207:
- Qstn #1Discuss the general characteristics of Group 15 elements with reference to their electronic configuration, oxidation state, atomic size, ionisation enthalpy and electronegativity.
Ans : General trends in group 15 elements
(i) Electronic configuration: All the elements in group 15 have 5 valence electrons. Their general electronic configuration is ns2 np3.
(ii) Oxidation states: All these elements have 5 valence electrons and require three more electrons to complete their octets. However, gaining electrons is very difficult as the nucleus will have to attract three more electrons. This can take place only with nitrogen as it is the smallest in size and the distance between the nucleus and the valence shell is relatively small. The remaining elements of this group show a formal oxidation state of -3 in their covalent compounds. In addition to the -3 state, N and P also show -1 and -2 oxidation states.
All the elements present in this group show +3 and +5 oxidation states. However, the stability of +5 oxidation state decreases down a group, whereas the stability of +3 oxidation state increases. This happens because of the inert pair effect.
(iii) Ionization energy and electronegativity
First ionization decreases on moving down a group. This is because of increasing atomic sizes. As we move down a group, electronegativity decreases, owing to an increase in size.
(iv) Atomic size: On moving down a group, the atomic size increases. This increase in the atomic size is attributed to an increase in the number of shells.
- Qstn #2Why does the reactivity of nitrogen differ from phosphorus?
Ans : Nitrogen is chemically less reactive. This is because of the high stability of its molecule, N2. In N2, the two nitrogen atoms form a triple bond. This triple bond has very high bond strength, which is very difficult to break. It is because of nitrogen’s small size that it is able to form p``\pi``-p``\pi`` bonds with itself. This property is not exhibited by atoms such as phosphorus. Thus, phosphorus is more reactive than nitrogen.
- Qstn #3Discuss the trends in chemical reactivity of group 15 elements.
Ans : General trends in chemical properties of group - 15
(i) Reactivity towards hydrogen:
The elements of group 15 react with hydrogen to form hydrides of type EH3, where E = N, P, As, Sb, or Bi. The stability of hydrides decreases on moving down from NH3 to BiH3.
(ii) Reactivity towards oxygen:
The elements of group 15 form two types of oxides: E2O3 and E2O5, where E = N, P, As, Sb, or Bi. The oxide with the element in the higher oxidation state is more acidic than the other. However, the acidic character decreases on moving down a group.
(iii) Reactivity towards halogens:
The group 15 elements react with halogens to form two series of salts: EX3 and EX5. However, nitrogen does not form NX5 as it lacks the d-orbital. All trihalides (except NX3) are stable.
(iv) Reactivity towards metals:
The group 15 elements react with metals to form binary compounds in which metals exhibit -3 oxidation states.
- Qstn #4Why does NH3 form hydrogen bond but PH3 does not?
Ans : Nitrogen is highly electronegative as compared to phosphorus. This causes a greater attraction of electrons towards nitrogen in NH3 than towards phosphorus in PH3. Hence, the extent of hydrogen bonding in PH3 is very less as compared to NH3.
- Qstn #5How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions involved.
Ans : An aqueous solution of ammonium chloride is treated with sodium nitrite.
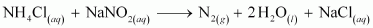
NO and HNO3 are produced in small amounts. These are impurities that can be removed on passing nitrogen gas through aqueous sulphuric acid, containing potassium dichromate.
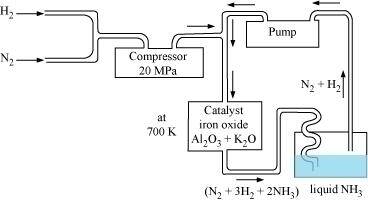
- Qstn #6How is ammonia manufactured industrially?
Ans : Ammonia is prepared on a large-scale by the Haber’s process.
$$\ce{N2(g)+3H2(g)-> 2NH3(g)}$$
``Δf_H°=-46.1kJ/mol``
The optimum conditions for manufacturing ammonia are:
(i) Pressure (around 200 × 105 Pa)
(ii) Temperature (700 K)
(iii) Catalyst such as iron oxide with small amounts of Al2O3 and K2O