NEET-XII-Chemistry
07: The p-Block Elements
- #6How is ammonia manufactured industrially?
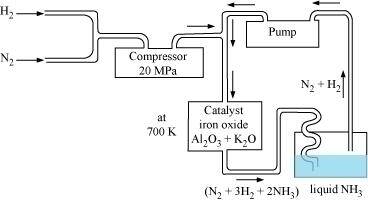
Ans : Ammonia is prepared on a large-scale by the Haber’s process.
$$\ce{N2(g)+3H2(g)-> 2NH3(g)}$$
``Δf_H°=-46.1kJ/mol``
The optimum conditions for manufacturing ammonia are:
(i) Pressure (around 200 × 105 Pa)
(ii) Temperature (700 K)
(iii) Catalyst such as iron oxide with small amounts of Al2O3 and K2O