NEET-XII-Chemistry
07: The p-Block Elements
- Qstn #14Write the order of thermal stability of the hydrides of Group 16 elements.
Ans : The thermal stability of hydrides decreases on moving down the group. This is due to a decrease in the bond dissociation enthalpy (H-E) of hydrides on moving down the group.
Therefore,

- Qstn #15Why is H2O a liquid and H2S a gas?
Ans : H2O has oxygen as the central atom. Oxygen has smaller size and higher electronegativity as compared to sulphur. Therefore, there is extensive hydrogen bonding in H2O, which is absent in H2S. Molecules of H2S are held together only by weak van der Waal’s forces of attraction.
Hence, H2O exists as a liquid while H2S as a gas.
- Qstn #16Which of the following does not react with oxygen directly?
Zn, Ti, Pt, Fe
Ans : Pt is a noble metal and does not react very easily. All other elements, Zn, Ti, Fe, are quite reactive. Hence, oxygen does not react with platinum (Pt) directly.
- Qstn #18Why does O3 act as a powerful oxidising agent?
Ans : Ozone is not a very stable compound under normal conditions and decomposes readily on heating to give a molecule of oxygen and nascent oxygen. Nascent oxygen, being a free radical, is very reactive.
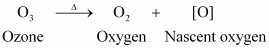
Therefore, ozone acts as a powerful oxidising agent.
- Qstn #19How is O3 estimated quantitatively?
Ans : Quantitatively, ozone can be estimated with the help of potassium iodide. When ozone is made to react with potassium iodide solution buffered with a borate buffer (pH 9.2), iodine is liberated. This liberated iodine can be titrated against a standard solution of sodium thiosulphate using starch as an indicator. The reactions involved in the process are given below.
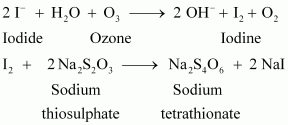
- Qstn #20What happens when sulphur dioxide is passed through an aqueous solution of Fe(III) salt?
Ans : SO2 acts as a reducing agent when passed through an aqueous solution containing Fe(III) salt. It reduces Fe(III) to Fe(II) i.e., ferric ions to ferrous ions.

- Qstn #21Comment on the nature of two S-O bonds formed in SO2 molecule. Are the two S-O bonds in this molecule equal?
Ans : The electronic configuration of S is 1s2 2s2 2p6 3s2 3p4.
During the formation of SO2, one electron from 3p orbital goes to the 3d orbital and S undergoes sp2 hybridization. Two of these orbitals form sigma bonds with two oxygen atoms and the third contains a lone pair. p-orbital and d-orbital contain an unpaired electron each. One of these electrons forms p``\pi``- p``\pi`` bond with one oxygen atom and the other forms p``\pi``- d``\pi`` bond with the other oxygen. This is the reason SO2 has a bent structure. Also, it is a resonance hybrid of structures I and II.
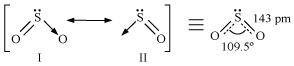
Both S-O bonds are equal in length (143 pm) and have a multiple bond character.
- Qstn #22How is the presence of SO2 detected?
Ans : SO2 is a colourless and pungent smelling gas.
It can be detected with the help of potassium permanganate solution. When SO2 is passed through an acidified potassium permanganate solution, it decolonizes the solution as it reduces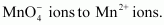

- Qstn #23Mention three areas in which H2SO4 plays an important role.
Ans : Sulphuric acid is an important industrial chemical and is used for a lot of purposes. Some important uses of sulphuric acid are given below.
(i) It is used in fertilizer industry. It is used to make various fertilizers such as ammonium sulphate and calcium super phosphate.
(ii) It is used in the manufacture of pigments, paints, and detergents.
(iii) It is used in the manufacture of storage batteries.
- Qstn #24Write the conditions to maximize the yield of H2SO4 by Contact process.
Ans : Manufacture of sulphuric acid by Contact process involves three steps.
1. Burning of ores to form SO2
2. Conversion of SO2 to SO3 by the reaction of the former with O2
(V2O5 is used in this process as a catalyst.)
3. Absorption of SO3 in H2SO4 to give oleum (H2S2O7)
The key step in this process is the second step. In this step, two moles of gaseous reactants combine to give one mole of gaseous product. Also, this reaction is exothermic. Thus, in accordance with Le Chatelier’s principle, to obtain the maximum amount of SO3 gas, temperature should be low and pressure should be high.
- Qstn #25Why is
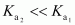 for H2SO4 in water?
for H2SO4 in water?
Ans :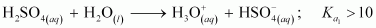
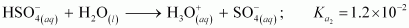
It can be noticed that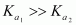
This is because a neutral H2SO4 has a much higher tendency to lose a proton than the negatively charged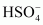 . Thus, the former is a much stronger acid than the latter.
. Thus, the former is a much stronger acid than the latter.
- Qstn #26Considering the parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy, compare the oxidising power of F2 and Cl2.
Ans : Fluorine is a much stronger oxidizing agent than chlorine. The oxidizing power depends on three factors.
1. Bond dissociation energy
2. Electron gain enthalpy
3. Hydration enthalpy
The electron gain enthalpy of chlorine is more negative than that of fluorine. However, the bond dissociation energy of fluorine is much lesser than that of chlorine. Also, because of its small size, the hydration energy of fluorine is much higher than that of chlorine. Therefore, the latter two factors more than compensate for the less negative electron gain enthalpy of fluorine. Thus, fluorine is a much stronger oxidizing agent than chlorine.