NEET-XII-Chemistry
07: The p-Block Elements
- #21Comment on the nature of two S-O bonds formed in SO2 molecule. Are the two S-O bonds in this molecule equal?
Ans : The electronic configuration of S is 1s2 2s2 2p6 3s2 3p4.
During the formation of SO2, one electron from 3p orbital goes to the 3d orbital and S undergoes sp2 hybridization. Two of these orbitals form sigma bonds with two oxygen atoms and the third contains a lone pair. p-orbital and d-orbital contain an unpaired electron each. One of these electrons forms p``\pi``- p``\pi`` bond with one oxygen atom and the other forms p``\pi``- d``\pi`` bond with the other oxygen. This is the reason SO2 has a bent structure. Also, it is a resonance hybrid of structures I and II.
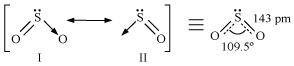
Both S-O bonds are equal in length (143 pm) and have a multiple bond character.