NEET-XII-Chemistry
02: Solutions
- #40Determine
the amount of CaCl2 (i =
2.47) dissolved in 2.5 litre of water such that its osmotic pressure
is 0.75 atm at 27°C.Ans : We
know that,

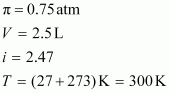
Here,
R
= 0.0821 L atm K-1mol-1
M
= 1 × 40 + 2 × 35.5
= 111g mol-1
Therefore, w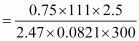
= 3.42 g
Hence,
the required amount of CaCl2 is 3.42 g.