Loading…
NEET-XII-Chemistry
02: Solutions
- #39The air is
a mixture of a number of gases. The major components are oxygen
and
nitrogen with approximate proportion of 20% is to 79% by volume at
298
K.
The water is in equilibrium with air at a pressure of 10 atm. At 298
Kif the
Henry’s
law constants for oxygen and nitrogen are 3.30 × 107 mm and 6.51 × 107 mm respectively, calculate the composition of these gases in water.Ans : Percentage
of oxygen (O2)
in air = 20 %
Percentage
of nitrogen (N2)
in air = 79%
Also, it
is given that water is in equilibrium with air at a total pressure of
10 atm, that is, (10 × 760) mm Hg = 7600 mm Hg
Therefore,
Partial
pressure of oxygen,
= 1520
mm Hg
Partial
pressure of nitrogen,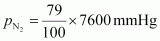
=
6004 mmHg
Now,
according to Henry’s law:
p = KH.x
For
oxygen:

For
nitrogen:

Hence,
the mole fractions of oxygen and nitrogen in water are 4.61 ×10-5and
9.22 × 10-5 respectively.