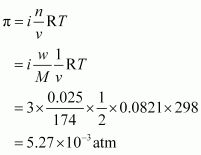NEET-XII-Chemistry
02: Solutions
Qstn# B-41 Prvs-Qstn
- #41Determine
the osmotic pressure of a solution prepared by dissolving 25 mg of
K2SO4 in 2 liter of water at 25° C, assuming that it is completely
dissociated.Ans : When K2SO4 is dissolved in water, ions are produced.
ions are produced.
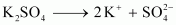
Total number of ions
produced = 3
 i =3
i =3
Given,
w = 25 mg =
0.025 g
V = 2 L
T = 250C
= (25 + 273) K = 298 K
Also, we know that:
R = 0.0821 L atm
K-1mol-1
M = (2 ×
39) + (1 × 32) + (4 × 16) = 174 g mol-1
Appling the following
relation,