Loading…
NEET-XII-Chemistry
02: Solutions
- #3319.5
g of CH2FCOOH
is dissolved in 500 g of water. The depression in the freezing point
of water observed is 1.0°C. Calculate the van’t Hoff factor
and dissociation constant of fluoroacetic acid.Ans : It is
given that:

We know
that:
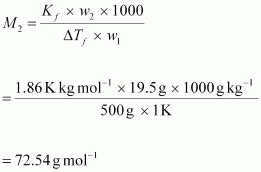
Therefore,
observed molar mass of
The
calculated molar mass of is:
is:
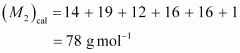
Therefore,
van’t Hoff factor,
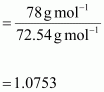
Let
α
be the degree of dissociation of


Now,
the value of Ka is given as:


Taking
the volume of the solution as 500 mL, we have the concentration:

Therefore,

