NEET-XII-Chemistry
02: Solutions
- #34Vapour
pressure of water at 293 Kis 17.535 mm Hg. Calculate the
vapour pressure of water at 293 Kwhen 25 g of glucose is dissolved in
450 g of water.Ans : Vapour pressure of
water, =
=
17.535 mm of Hg
Mass of glucose, w2 = 25 g
Mass of water, w1 = 450 g
We know that,
Molar mass of glucose
(C6H12O6), M2 = 6
× 12 + 12 × 1 + 6 × 16
= 180 g mol-1
Molar mass of water, M1 = 18 g mol-1
Then, number of moles
of glucose,
= 0.139 mol
And, number of moles of
water,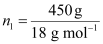
= 25 mol
We know that,
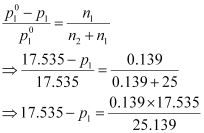
⇒ 17.535 - p1 = 0.097
⇒ p1 = 17.44 mm of Hg
Hence, the vapour
pressure of water is 17.44 mm of Hg.