NEET-XII-Chemistry
02: Solutions
- #32Calculate
the depression in the freezing point of water when 10 g of
CH3CH2CHClCOOH
is added to 250 g of water. Ka = 1.4 × 10-3, Kf = 1.86
K kg
mol-1.Ans : Molar
mass of

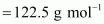
∴No.
of moles present in 10 g of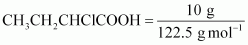

It
is given that 10 g of is
is
added to 250 g of water.
∴Molality
of the solution,

Let α be the degree of dissociation of
 undergoes
undergoes
dissociation according to the following equation:


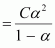
Since α is very small with respect to 1, 1 - α
≈
1
Now,
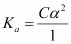
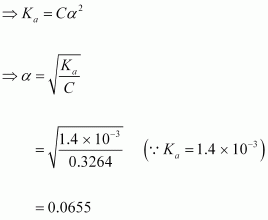
Again,

Total
moles of equilibrium = 1 - α
+ α
+ α
= 1
+ α


Hence, the
depression in the freezing point of water is given as:

