NEET-XII-Chemistry
02: Solutions
- #31The
depression in freezing point of water observed for the same amount of
acetic acid, trichloroacetic acid and trifluoroacetic acid increases
in the order given above. Explain briefly.Ans :

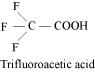
Among H, Cl, and F, H
is least electronegative while F is most electronegative. Then, F can
withdraw electrons towards itself more than Cl and H. Thus,
trifluoroacetic acid can easily lose H+ ions i.e.,
trifluoroacetic acid ionizes to the largest extent. Now, the more
ions produced, the greater is the depression of the freezing point.
Hence, the depression in the freezing point increases in the order:
Acetic acid <
trichloroacetic acid < trifluoroacetic acid