NEET-XII-Chemistry
02: Solutions
- #20A
5% solution (by mass) of cane sugar in water has freezing point of
271 K.
Calculate the freezing point of 5% glucose in water if freezing point
of pure water is 273.15 K.Ans : Here, ΔTf = (273.15 - 271) K
= 2.15 K
Molar mass of sugar
(C12H22O11) = 12 × 12 + 22 ×
1 + 11 × 16
= 342 g mol-1
5% solution (by mass)
of cane sugar in water means 5 g of cane sugar is present in (100 -
5)g = 95 g of water.
Now, number of moles of
cane sugar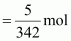
= 0.0146 mol
Therefore, molality of
the solution,
= 0.1537 mol kg-1
Applying the relation,
ΔTf = Kf × m
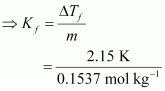
= 13.99 K kg
mol-1
Molar of glucose
(C6H12O6) = 6 × 12 + 12 ×
1 + 6 × 16
= 180 g mol-1
5% glucose in water
means 5 g of glucose is present in (100 - 5) g = 95 g of water.
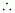 Number of moles of glucose
Number of moles of glucose 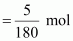
= 0.0278 mol
Therefore, molality of
the solution,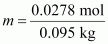
= 0.2926 mol kg-1
Applying the relation,
ΔTf = Kf × m
= 13.99 K kg mol-1 × 0.2926 mol kg-1
= 4.09 K
(approximately)
Hence, the freezing
point of 5% glucose solution is (273.15 - 4.09) K= 269.06 K.