NEET-XII-Chemistry
02: Solutions
- #21Two
elements A and B form compounds having formula AB2 and AB4.
When dissolved in 20 g of benzene (C6H6),
1 g of AB2 lowers the freezing point by 2.3 Kwhereas 1.0 g of AB4 lowers it by 1.3 K.
The molar depression constant for benzene is 5.1 Kkg mol-1.
Calculate atomic masses of A and B.Ans : We know that,
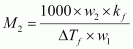
Then,
= 110.87 g mol-1

= 196.15 g mol-1
Now, we have the molar
masses of AB2 and AB4 as 110.87 g mol-1 and 196.15 g mol-1 respectively.
Let
the atomic masses of A and B be x and y respectively.
Now,
we can write:
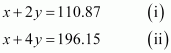
Subtracting equation
(i) from (ii), we have
2y = 85.28
⇒ y =
42.64
Putting the value of
‘y’ in equation (1), we have
x + 2 ×
42.64 = 110.87
⇒ x =
25.59
Hence, the atomic
masses of A and B are 25.59 u and 42.64 u respectively.
Page No 61: