NEET-XII-Chemistry
02: Solutions
- #19A solution
containing 30 g of non-volatile solute exactly in 90 g of water has a
vapour
pressure of 2.8 kPa at 298 K.
Further, 18 g of water is then added to
the
solution and the new vapour pressure becomes 2.9 kPa at 298 K.
Calculate:
- molar
mass of the solute - vapour
pressure of water at 298 K.
Ans : (i) Let,
the molar mass of the solute be M g mol-1
Now,
the no. of moles of solvent (water),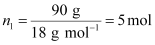
And,
the no. of moles of solute,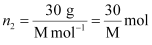
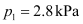
Applying the relation:
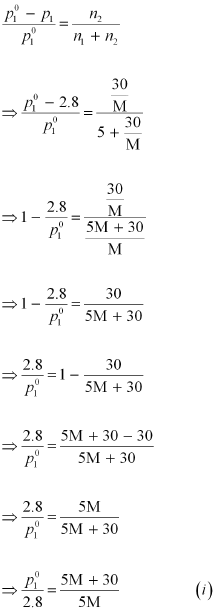
After
the addition of 18 g of water:
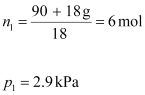
Again, applying the relation:
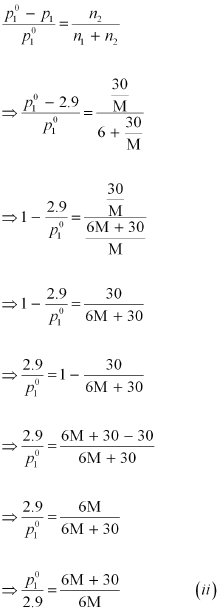
Dividing
equation (i) by (ii),
we have:
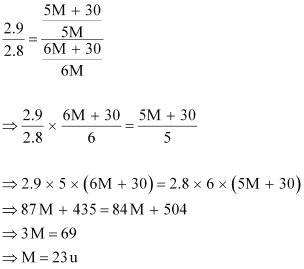
Therefore,
the molar mass of the solute is 23 g mol-1.
(ii) Putting
the value of ‘M’ in equation (i),
we have:
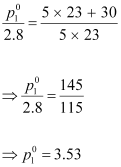
Hence, the vapour pressure of water at 298 K is 3.53 kPa. - molar