NEET-XII-Chemistry
02: Solutions
- #18Calculate
the mass of a non-volatile solute (molar mass 40 g mol-1)
which should be dissolved in 114 g octane to reduce its vapour
pressure to 80%.Ans : Let the vapour pressure
of pure octane be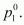
Then, the vapour
pressure of the octane after dissolving the non-volatile solute is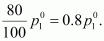
Molar mass of solute, M2 = 40 g mol-1
Mass of octane, w1 = 114 g
Molar mass of octane,
(C8H18), M1 = 8 × 12 +
18 × 1
= 114 g mol-1
Applying the relation,
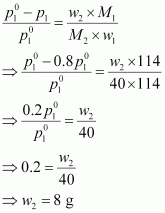
Hence, the required
mass of the solute is 8 g.