NEET-XII-Chemistry
02: Solutions
- #17The
vapour pressure of water is 12.3 kPa at 300 K.
Calculate vapour pressure of 1 molal solution of a non-volatile
solute in it.Ans : 1 molal solution means 1 mol of the solute is present in 1000 g of the solvent (water).
Molar mass of water = 18 g mol-1
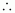 Number of moles present in 1000 g of water
Number of moles present in 1000 g of water 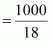
= 55.56 mol
Therefore, mole fraction of the solute in the solution is
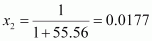 .
.
It is given that,
Vapour pressure of water,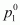 = 12.3 kPa
= 12.3 kPa
Applying the relation,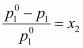
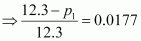
⇒ 12.3 - p1 = 0.2177
⇒ p1 = 12.0823
= 12.08 kPa (approximately)
Hence, the vapour pressure of the solution is 12.08 kPa.