NEET-XII-Chemistry
02: Solutions
- #16Heptane
and octane form an ideal solution. At 373 K,
the vapour pressures of the two liquid components are 105.2 kPa and
46.8 kPa respectively. What will be the vapour pressure of a mixture
of 26.0 g of heptane and 35 g of octane?Ans : Vapour pressure of
heptane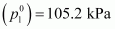
Vapour pressure of
octane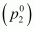 =
=
46.8 kPa
We know that,
Molar mass of heptane
(C7H16) = 7 × 12 + 16 × 1
= 100 g mol-1
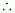 Number
Number
of moles of heptane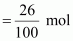
= 0.26 mol
Molar mass of octane
(C8H18) = 8 × 12 + 18 × 1
= 114 g mol-1
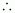 Number
Number
of moles of octane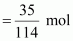
= 0.31 mol
Mole fraction of
heptane,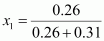
= 0.456
And, mole fraction of
octane, x2 = 1 - 0.456
= 0.544
Now, partial pressure
of heptane,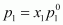
= 0.456 × 105.2
= 47.97 kPa
Partial pressure of
octane,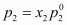
= 0.544 × 46.8
= 25.46 kPa
Hence, vapour pressure
of solution, ptotal = p1 + p2
= 47.97 + 25.46
= 73.43 kPa