NEET-XII-Chemistry
02: Solutions
- #15An
aqueous solution of 2% non-volatile solute exerts a pressure of 1.004
bar at the normal boiling point of the solvent. What is the molar
mass of the solute?Ans : Here,
Vapour pressure of the
solution at normal boiling point (p1) = 1.004 bar
Vapour pressure of pure
water at normal boiling point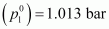
Mass of solute, (w2)
= 2 g
Mass of solvent
(water), (w1) = 98 g
Molar mass of solvent
(water), (M1) = 18 g mol-1
According to Raoult’s
law,
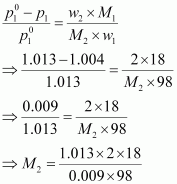
= 41.35 g mol-1
Hence, the molar mass
of the solute is 41.35 g mol-1.