NEET-XII-Chemistry
Previous Year Paper year:2018
- Qstn #46A mixture of 2.3 g formic acid and 4.5 g
oxalic acid is treated with conc. ``H_2SO_4``.
The evolved gaseous mixture is passed through
KOH pellets. Weight (in g) of the remaining
product at STP will be
(1) 1.4
(2) 3.0
(3) 2.8
(4) 4.4digAnsr: 3Ans : (3)
Sol.
HCOOH 2 4H SO
Dehydrating Agent
CO + H2O
H O abosrbed
by H SO
2
2 4
(moles)i =
2.3 1
46 20
= 0 0
(moles)f 0
1
20
1
20
H2C2O4 2 4H SO CO + CO2 + H2O
[H2O absorbed by H2SO4]
(moles)i
4.5 1
90 20
= 0 0 0
(moles)f 0
1
20
1
20
1
20
CO2 is absorbed by KOH.
So the remaning product is only CO.
moles of CO formed from both reactions
=
1 1
20 20
+ =
1
10
Left mass of CO = moles × molar mass
=
1
28
10
= 2.8 g Ans.
- Qstn #47Nitration of aniline in strong acidic medium also
gives m-nitroaniline because
(1) In spite of substituents nitro group always
goes to only m-position.
(2) In electrophilic substitution reactions amino
group is meta directive.
(3) In absence of substituents nitro group always
goes to m-position
(4) In acidic (strong) medium aniline is present as
anilinium ion.digAnsr: 4Ans : (4)
Sol.
NH2
H
nitrating
mixture
NH3
NO2
NH3
NO2
In acidic medium aniline is protonated to form
anilinium ion which is metadirecting.
- Qstn #48Which of the following oxides is most acidic in
nature?
(1) MgO
(2) BeO
(3) BaO
(4) CaOdigAnsr: 2Ans : (2)
Sol. In metals moving down the group metallic character
increases, so basic nature increases hence most
acidic will be BeO.
- Qstn #49The difference between amylose and amylopectin is
(1) Amylopectin have 1 ``\rightarrow``4 ``\alpha``-linkage and 1 ``\rightarrow`` 6
``\alpha``-linkage
(2) Amylose have 1 ``\rightarrow`` 4 ``\alpha``-linkage and 1 ``\rightarrow`` 6
``\alpha``-linkage
(3) Amylopectin have 1 ``\rightarrow`` 4 ``\alpha``-linkage and 1 ``\rightarrow`` 6
``\alpha``-linkage.
(4) Amylose is made up of glucose and galactose.digAnsr: 1Ans : (1)
Sol.
Amylose is long unbranched chain with
-D-Glucose with held by C1-C4 glucosidic linkage
whereas amylopectin is branched chain polymer of
-D glucose unit in which chain is formed by
C1-C4 glycosidic linkage while branching occurs by
C1-C6 glucosidic linkage.
- Qstn #50Regarding cross-linked or network polymers, which
of the following statements is incorrect?
(1) They contain covalent bonds between various
linear polymer chains.
(2) They are formed from bi-and tri-functional
monomers.
(3) Examples are bakelite and melamine.
(4) They contain strong covalent bonds in their
polymer chains.digAnsr: 4Ans : (4)
Sol. Cross-linked or network polymers are usually
formed from bi-functional & tri-functional
monomers and contains strong covalent bond
between various linear polymer chains like
Melamine, Bakelite etc.
CHEMISTRY
11
CODE - PP
- Qstn #51In the reaction
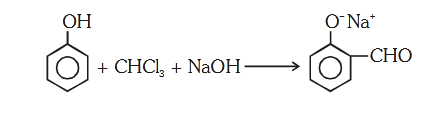
the electrophile involved is
(1) dichloromethyl cation ``\ce{(CHCl2)^\oplus}``
(2) formyl cation ``\ce{(CHO)^\oplus}``
(3) dichloromethyl anion ``\ce{(CHCl2)^\ominus}``
(4)dichlorocarbene ``\ce{(:CCl2)}``digAnsr: 4Ans : (4)
Sol.
OH
CHCl3
NaOH
O Na
CHCl + NaOH 3 CCl + H O3 2
-Cl ( -Elimination)
:CCl dichlorocarbene
(electrophile)
2
CHO
- Qstn #52Carboxylic acid have higher boiling points than
aldehydes, ketones and even alcohols of
comparable molecular mass. It is due to their
(1) formation of intramolecular H-bonding
(2) formation of carboxylate ion
(3) more extensive association of carboxylic acid via
van der Waals force of attraction
(4) formation of intermolecular H-bonding.digAnsr: 4Ans : (4)
Sol. Carboxylic acid has higher boiling point than
aldehyde, ketone and even alcohols of comparable
molecular mass.
This is due to more extensive association through
intermolecular H-bonding.
R-C C-R
O
O-H O
H-O
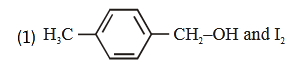
- Qstn #53Compound A, ``\ce{(C8H10O)}``, is found to react with NaOI
(produced by reacting Y with NaOH) and yields a
yellow precipitate with characteristic smell.
A and Y are respectively
(1)
(2)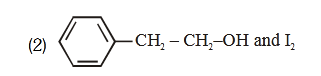
(3)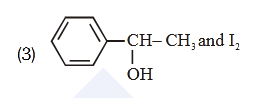
(4)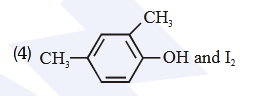 digAnsr: 3Ans : (3)
digAnsr: 3Ans : (3)
Sol. Haloform reaction is shown by compound having
CH -C- or CH -CH-3 3
O OH
Group
-CH-CH3
OH
NaOI or
NaOH + I2
-C-ONa + CHI3
O yellow ppt
- Qstn #54The correct difference between first- and
second-order reaction is that
(1) the rate of a first-order reaction does not depend
on reactant concentration; the rate of a second-
order reaction does depend on reactant
concentrations.
(2) the half-life of a first-order reaction does not
depend on ``[A]_0``; the half-life of a second-order
reaction does depend on ``[A]_0``
(3) a first-order reaction can be catalyzed;
a second-order reaction cannot be catalyzed.
(4) the rate of a first-order reaction does depend
on reactant concentrations; the rate of a
second-order reaction does not depend on
reactant concentrationsdigAnsr: 2Ans : (2)
Sol. (t1/2)1st order = Independent of Concentration
(t1/2)2
nd order 0
1
A
12
- Qstn #55Among ``\ce{CaH2}``, ``\ce{BeH2}``, ``\ce{BaH2}``, the order of ionic
character is
(1)``\ce BeH2 < CaH2 < BaH2``
(2) ``\ce CaH2 < BeH2 < BaH2``
(3) ``\ce BeH2 < BaH2 < CaH2``
(4) ``\ce BaH2 < BeH2 < CaH2``digAnsr: 1Ans : (1)
Sol. BeH2 < CaH2 < BaH2
Smaller the size of cation, more will be its polarising
power. Hence BeH2 will be least ionic.
- Qstn #56Consider the change in oxidation state of Bromine
corresponding to different emf values as shown in
the diagram below:
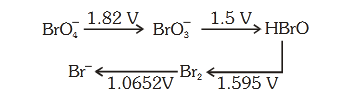
Then the species undergoing disproportionation is:-
(1) ``\ce{BrO3-}``
(2) ``\ce{BrO4-}``
(3) ``\ce{Br2}``
(4) HBrOdigAnsr: 4Ans : (4)
Sol. Calculate E°cell corresponding to each compound
under going disproportionation reaction. The
reaction for which E°cell comes out +ve is
spontaneous.
HBrO Br2 E° = 1.595, SRP (cathode)
HBrO BrO3
- E° = -1.5V, SOP (Anode)
2HBrO Br2 + BrO3
-
E°cell = SRP (cathode) - SRP (Anode)
= 1.595 - 1.5
= 0.095 V
E°cell > 0 &implies; ▵G° < 0 [spontaneous]
- Qstn #57In which case is the number of molecules of
water maximum?
(1) 18 mL of water
(2) 0.18 g of water
(3) 0.00224 L of water vapours at 1 atm and 273 K
(4) ``10^{-3}`` mol of waterdigAnsr: 1Ans : (1)
Sol. (1) 18 mL water
As dH2O = 1 g/mL So WH2O = 18g
nH2O =
18
1
18
=
molecules = 1 × NA
(2) 0.18 g of water
nH2O =
0.18
18
= 0.01
(molecules)H2O = 0.01 × NA
(3) (VH2O(g))STP = 0.00224 L
nH2O =
V 0.00224
22.4 22.4
= = 0.0001
molecules = 0.0001 × NA
(4) nH2O = 10
-3
(molecules)H2O = 10
-3 × NA
- Qstn #58Magnesium reacts with an element (X) to form an
ionic compound. If the ground state electronic
configuration of (X) is ``1s^2`` ``2s^2`` ``2p^3``, the simplest
formula for this compound is
(1) ``\ce{Mg2X3}`` (2) ``\ce{MgX2}``
(3)``\ce{Mg2X}`` (4)``\ce{Mg3X2}``digAnsr: 4Ans : (4)
Sol. Magnesium ion = Mg+2
X = Nitrogen Nitrogen ion = N-3
Mg+2 N-3
Mg N3 2/(Mg X )3 2
- Qstn #59Iron exhibits bcc structure at room temperature.
Above 900°C, it transforms to fcc structure. The
ratio of density of iron at room temperature to that
at 900°C (assuming molar mass and atomic radii of
iron remains constant with temperature) is
(1)``\frac{\sqrt3}{\sqrt2}``
(2)``\frac{4\sqrt3}{3\sqrt2}``
(3)``\frac{3\sqrt3}{4\sqrt2}``
(4)``\frac{1}{2}``digAnsr: 3Ans : (3)
Sol. BCC FCC
4r = 3a 4r = 2a
a =
4r
3
a =
4r
2
BCC
FCC
d
d =
BCC
3
A
FCC
3
A
Z M
N a
Z M
N a
=
3
A
3
A
2 M
4r
N
3
4 M
4r
N
2
=
3 3
4 2