ICSE-X-Chemistry
Previous Year Paper year:2014
- #1-e-iiiAction of concentrated sulphuric acid on carbon
- #1-e-ivAction of dilute hydrochloric acid on sodium sulphide
- #1-e-v [5]Preparation of ethane from sodium propionate
- #1-fDistinguish between the following pairs of compounds using the test given within
brackets:
- #1-f-iIron (II) sulphate and iron (III) sulphate (using ammonium hydroxide)Ans : Iron II sulphate: Gives dirty green ppt with ammonium hydroxide insoluble in excess.
Iron III sulphate: Gives reddish brown ppt with ammonium hydroxide insoluble in excess.
- #1-f-iiA lead salt and a zinc salt (using excess ammonium hydroxide)Ans : Lead salt: Gives white ppt with ammonium hydroxide which is insoluble in excess.
Zinc salt: Gives gelatenous white ppt which is soluble in excess ammonium hydroxide.
- #1-f-iiiSodium nitrate and sodium sulphite (using dilute sulphuric acid)Ans : Sodium nitrate: Colourless vapours of nitric acid which condenses to form nitric acid.
Sodium sulphite: Colourless, gas with smell of burning sulphur, acidic in nature that is sulphur di oxide is released.
- #1-f-ivDilute sulphuric acid and dilute hydrochloric acid (using barium chloride solution)Ans : With dil. HCl, BaCl2 gives no ppt with dil. H2SO4, BaCl2 gives a white insoluble ppt of BaSO4.
- #1-f-v [5]Ethane and ethene (using alkaline potassium permanganate solution)Ans : With ethane, purple colour of potassium permanganate remains unfaded with ethene the purple colour gets decolourised.
- #1-gAns :
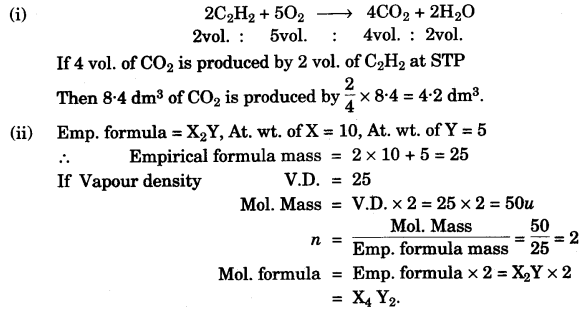
- #1-g-iOxygen oxidises ethyne to carbon dioxide and water as shown by the equation:
$$\ce{2C2H2 + 5O2 -> 4CO2 + 2H2O }$$
What volume of ethyne gas at STP is required to produce ``\pu{8.4 dm3}`` of carbon
dioxide at STP? [H = 1, C = 12, O = 16]
- #1-g-ii [5]A compound made up of two elements X and Y has an empirical formula ``\ce{X2Y}``. If the
atomic weight of X is 10 and that of Y is 5 and the compound ha a vapour density 25,
find its molecular formula.
- # [40]Section : IIAttempt any four questions from this section.
- #2
- #2-aState your observation in each of the following cases: