ICSE-X-Chemistry
Previous Year Paper year:2014
- #2-a-iWhen dilute hydrochloric acid is added to sodium carbonate crystals.Ans : i. Brisk effervescence with the release of a colourless odourless gas that extinguish a glowing splint and turns lime water milky i.e., CO2 gas is released.
- #2-a-iiWhen excess sodium hydroxide is added to calcium nitrate solution.Ans : ii. A white ppt of Ca(OH)2 is obtained that remains insoluble in excess of NaOH.
- #2-a-iiiAt the cathode, when acidified aqueous copper sulphate solution is electrolysed with
copper electrodes.Ans : iii. The blue colour of aq.CuSO4 remains unchanged.
- #2-a-ivWhen calcium hydroxide is heated with ammonium chloride crystals.Ans : iv. A colourless pungent smelling basic gas i.e., Ammonia is obtained.
- #2-a-v [5]When moist starch iodide paper is introduced into chlorine gas.Ans : v. Moist starch iodide paper turns blue black.
- #2-bStudy the figure given below and answer the questions which follow:
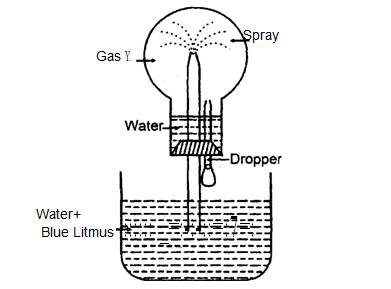
- #2-b-iIdentify the gas Y.Ans : i. Hydrogen chloride gas (HCl).
- #2-b-iiWhat property of gas Y does this experiment demonstrate?Ans : ii. Y Gas i.e., HCl gas is highly soluble and acidic in nature.
- #2-b-iii [3]Name another gas which has the same property and can be demonstrated through this
experiment.Ans : iii. Ammonia gas.
- #2-c
- #2-c-iName the other ion formed when ammonia dissolves in water.Ans : i. Hydroxyl ion (OH-) other than Ammonium ion.
- #2-c-ii [2]Give one test which can be used to detect the presence of the ion produced.Ans : ii. Red litmus turns blue/Methyl orange yellow/Phenolphthalein turns pink.
- #3
- #3-aState the conditions required for the following reactions to take place:
- #3-a-iCatalytic hydrogenation of ethyneAns : In presence of Catalyst like Ni/Pt/Pd etc.