ICSE-X-Chemistry
Previous Year Paper year:2009
- #2-a-ii. Lead bromide conducts electricity.Ans : Molten lead bromide conducts electricity.
- #2-a-iiii. Copper reacts with nitric acid to produce nitrogen dioxide.Ans : Copper reacts with concentrated nitric acid to produce nitrogen dioxide.
- #2-a-iiiiii. Haematite is the chief ore of aluminium.Ans : Bauxite is the chief ore of aluminium.
- #2-a-iviv. Equal masses of all gases under identical conditions contain the same number of
molecules.Ans : Equal volume of all gases under identical conditions contain the same number of molecules.
- #2-a-vv. Hydrochloric acid is prepared in the laboratory by passing hydrogen chloride
directly through water.Ans : Hydrochloric acid is prepared in the laboratory by passing hydrogen chloride gas through water by inverted funnel arrangement.
- #2-b [5]Consider the section of the periodic table given below:
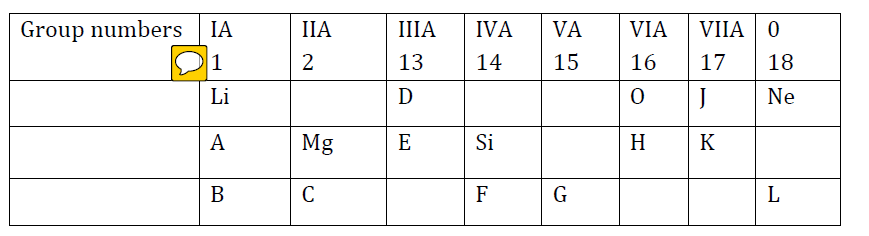
Note: In this table, B does not represent boron,
C does not represent carbon,
F does not represent fluorine,
H does not represent hydrogen,
K does not represent potassium.
You must see the position of the element in the periodic table.
Some elements are given in their own symbol and position in the periodic table, while
others are shown with a letter. With reference to the table:
- #2-b-ii. Which is the most electronegative?Ans : Element J
- #2-b-iiii. How many valence electrons are present in G?Ans : Five
- #2-b-iiiiii. Write the formula of the compound between B and H.Ans : B2H
- #2-b-iviv. In the compound between F and J, what type of bond will be formed?Ans : Covalent
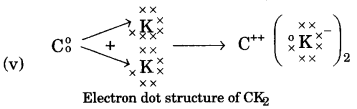
- #2-b-vv. Draw the electron dot structure for the compound formed between C and K.Ans :
- #3
- #3-a [5]A metal article is to be electroplated with silver. The electrolyte selected is sodium
argentocyanide.
- #3-a-ii. What kind of salt is sodium argentocyanide?Ans : A complex salt.
- #3-a-iiii. Why is it preferred to silver nitrate as an electrolyte?Ans : When silver nitrate is used, deposition of silver on cathode is not uniform because it is a strong electrolyte.