ICSE-X-Chemistry
Previous Year Paper year:2009
- #3-a-iiiiii. State one condition to ensure that the deposit is smooth, firm and long lasting.Ans : A small current is passed for a longer time.
- #3-a-iviv. Write the reaction taking place at the cathode.Ans : Ag+ + e- -> Ag
- #3-a-vv. Write the reaction taking place at the anode.Ans : Ag - e- -> Ag+.
- #3-b [3]The sketch below illustrates the refining of aluminium by Hoopes process.

- #3-b-ii. Which of A and B is the cathode and which one is the anode?Ans : A is cathode and B is anode.
- #3-b-iiii. What is the electrolyte in the tank?Ans : Molten fluorides.
- #3-b-iiiiii. What material is used for the cathode?Ans : Graphite rods dipped in pure molten aluminium.
- #3-c [2]State the property of the metal being utilised in the following:
Use of metal Property Zinc in galvanisation Aluminium in thermite welding Ans :Use of metal Property Zinc in Galvanization Zinc forms a protective layer of zinc oxide which prevents rusting of iron. Aluminium in Thermite welding Strong affinity for oxygen.
- #4
- #4-a [6]Ans :
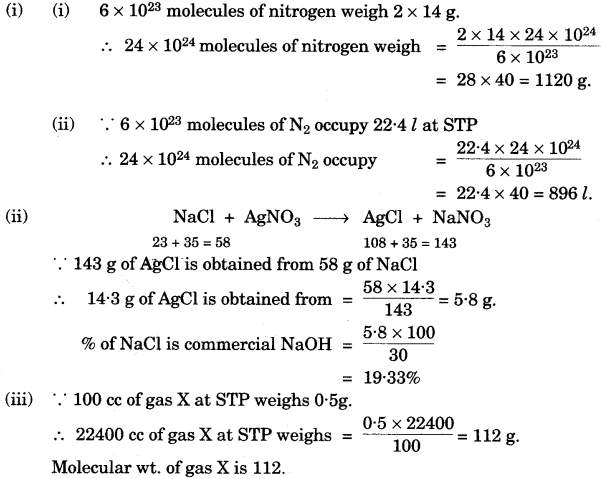
- #4-a-ii. A gas cylinder contains ``24 \times 10^{24}`` molecules of nitrogen gas. If Avogadro.s number is
``6 \times10^{23}`` and the relative atomic mass of nitrogen is 14, calculate:
1. Mass of nitrogen gas in the cylinder.
2. Volume of nitrogen at STP in ``\pu{dm3}``.
- #4-a-iiii. Commercial sodium hydroxide weighing ``\pu{30 g}`` has some sodium chloride in it. The
mixture on dissolving in water and subsequent treatment with excess silver nitrate
solution formed a precipitate weighing ``\pu{14.3 g}``. What is the percentage of sodium
chloride in the commercial sample of sodium hydroxide? The equation for the
reaction is
$$\ce{NaCl + AgNO3 . AgCl + NaNO3 }$$.
[Relative molecular mass of NaCl = 58; AgCl = 143]
- #4-a-iiiiii. A certain gas .X. occupies a volume of ``\pu{100 cm3}`` at STP and weighs 0.5 g. Find its
relative molecular mass.
- #4-b [4]Solution A is a strong acid.
Solution B is a weak acid.
Solution C is a strong alkali.
- #4-b-ii. Which solution contains solute molecules in addition to water molecules?Ans : Solution B