ICSE-X-Chemistry
Previous Year Paper year:2009
- #1-d-iviv. Magnesium nitride is treated with warm water.Ans : $$\ce{ Mg3N2 + 6H2O ->3Mg(OH)2 + 2NH3 }$$
- #1-d-vv. Acetic acid is warmed with ethanol in the presence of concentrated sulphuric acid.Ans : $$\ce{ CH3COOH + C2H5OH ->{CONC. H2SO4}{\delta} CH3COOC2H5 +H2O }$$
- #1-e [5]Find the odd one out and explain your choice (Note: Valency is not a criterion.):
i) $$\ce{ Al(OH)3, Pb(OH)2, Mg(OH)2, Zn(OH)2 }$$
ii) $$\ce{C3H8, C5H10, C2H6, CH4 }$$
iii) Sulphur, Phosphorus, Carbon, Iodine
iv) Copper, Lead, Zinc, Mercury
v) Formic acid, Nitric acid, Acetic acid, Propanoic acidAns :
(i) Mg(OH)2: It is basic while rest are amphoteric.
(ii) C5H10: It is an alkene while the rest are saturated hydrocarbons.
(iii) Carbon: It forms a very large number of compounds while rest do not.
(iv) Mercury: It is a liquid metal while rest are solid.
(v) Nitric acid: It is a mineral acid while rest are organic acids.
- #1-f [5]Identify the substances P, Q, R, S and T in each case based on the information given
below:
- #1-f-ii. The deliquescent salt P turns yellow on dissolving in water and gives a reddish
brown precipitate with sodium hydroxide solution.Ans : P is Ferric chloride.
- #1-f-iiii. The white crystalline solid Q is soluble in water. It liberates a pungent smelling gas
when heated with sodium hydroxide solution.Ans : Q is ammonium chloride.
- #1-f-iiiiii. The pale green solid R turns reddish brown on heating. Its aqueous solution gives a
white precipitate with barium chloride solution. The precipitate is insoluble in
mineral acids.Ans : R is Ferrous sulphate.
- #1-f-iviv. The reddish brown liquid S is dissolved in water. When ethyne gas is passed
through it, it turns colourless.Ans : S is bromine.
- #1-f-vv. The nitrate T does not leave any residue on heating. **Ans : **may be out of syllabus
- #1-g [5]Ans :
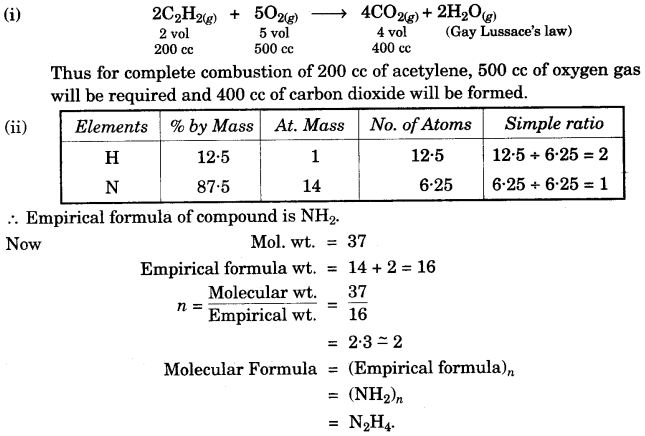
- #1-g-ii. Calcium carbide is used for the artificial ripening of fruits. Actually, the fruit ripens
because of the heat evolved, while calcium carbide reacts with moisture. During this
reaction, calcium hydroxide and acetylene gas are formed. If 200 cm3 of acetylene is
formed from a certain mass of calcium dioxide during the complete combustion,
then the combustion reaction can be represented as below:
$$\ce{ 2C2H2 (g) + 5O2 (g) -> 4CO2 (g) + 2H2O (g) }$$
- #1-g-iiii. A gaseous compound of nitrogen and hydrogen contains 12.5% hydrogen by mass.
Find the molecular formula of the compound if its relative molecular mass is 37.
[N = 14, H = 1]
- # [40]Section : IIAttempt any four questions from this section.
- #2
- #2-a [5]Correct the following statements.
For example: .Chlorine is a bleaching agent..
Should read as .Moist chlorine is a bleaching agent..