ICSE-X-Chemistry
Previous Year Paper year:2009
- #1-c [10]For part c (i).(x), select the correct answer from the choices A, B, C and D which are
given.
Write only the letter corresponding to the correct answer:
- #1-c-ii. Among the period 2 elements, the one which has high electron affinity is
(A) Lithium
(B) Carbon
(C) Fluorine
(D) NeonAns : (C)
- #1-c-iiii. Among the following, the one which is composed of all the three kinds of bonds
(ionic, covalent and coordinate bond) is
(A) Sodium chloride
(B) Ammonia
(C) Carbon tetrachloride
(D) Ammonium chlorideAns : (D)
- #1-c-iiiiii. Which of the following statements is wrong about alkanes?
(A) They are all saturated hydrocarbons.
(B) They can undergo addition as well as substitution reactions.
(C) They are almost non-polar in nature.
(D) On complete combustion give out carbon dioxide and water.Ans : (B)
- #1-c-iviv. Select the acid which contains four hydrogen atoms in it.
(A) Formic acid
(B) Sulphuric acid
(C) Nitric acid
(D) Acetic acidAns : (D)
- #1-c-ixix. The organic compound obtained as the end-product of the fermentation of sugar
solution is
(A) Methanol
(B) Ethanol
(C) Ethane
(D) Methanoic acidAns : (B)
- #1-c-vv. A gas cylinder of capacity of ``\pu{20 dm3 }`` is filled with gas X, the mass of which is 10g.
When the same cylinder is filled with hydrogen at the same temperature and
pressure, the mass of the hydrogen is 2 g; hence, the relative molecular mass of the
gas is
(A) 5
(B) 10
(C) 15
(D) 20Ans : (B)
- #1-c-vivi. The aqueous solution of the following compounds which contains both ions and
molecules is
(A) Sulphuric acid
(B) Hydrochloric acid
(C) Nitric acid
(D) Acetic acidAns : (D)
- #1-c-viivii. The metal oxide which can react with acid as well as alkali is
(A) Silver oxide
(B) Copper(II) oxide
(C) Aluminium oxide
(D) Calcium oxideAns : (C)
- #1-c-viiiviii. Carbon dioxide and sulphur dioxide gas can be distinguished by using
(A) Moist blue litmus paper
(B) Lime water
(C) Acidified potassium dichromate paper
(D) None of the aboveAns : (C)
**may be out of syllabus
- #1-c-xx. A black colour solid which on reaction with dilute sulphuric acid forms a blue
coloured solution is**
(A) Carbon
(B) Manganese(IV) oxide
(C) Lead(II) oxide
(D) Copper(II) oxideAns : (D)
- #1-d [5]Write a fully balanced equation for each of the following cases:Ans :
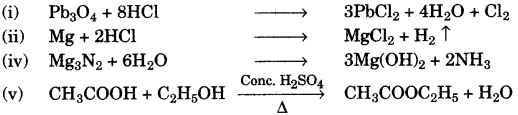
- #1-d-ii. Red lead is warmed with concentrated hydrochloric acid.Ans : $$\ce{ Pb3O4 + 8HCL -> 3PbCl2 + 4H2O + Cl2 }$$
- #1-d-iiii. Magnesium metal is treated with dilute hydrochloric acid.Ans : $$\ce{ Mg + 2HCl -> MgCl2 + H2(^) }$$
- #1-d-iiiiii. Lead nitrate is heated in a dry test tube.Ans : $$\ce{2 Pb(NO3)2 (s) -> 2 PbO(s) + 4 NO2(g) + O2(g) }$$