NEET-XII-Physics
26: Laws of Thermodynamics
- #21-aHow much work has been done by the gas on the left part? (b) Find the final pressures on the two sides. (c) Find the final equilibrium temperature. (d) How much heat has flown from the gas on the right to the gas on the left?
FigureAns : As the diathermic wall is fixed, so final volume of the chambers will be same. Thus, ΔV = 0, hence work done ΔW= PΔV = 0
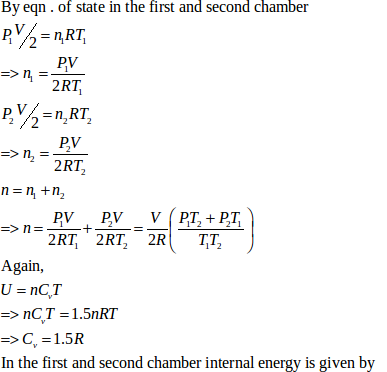
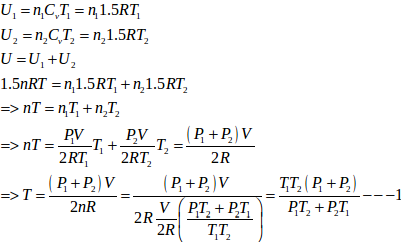
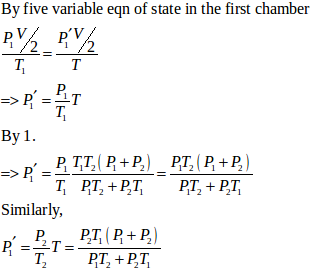
(b) Let final pressure in the first and second compartment P1’ and P2’.
 (c)
(c)
 (d)
(d) 
Page No 64:
- #21-bFind the final pressures on the two sides.Ans : Let final pressure in the first and second compartment P1’ and P2’.

- #21-cFind the final equilibrium temperature.Ans :

- #21-dHow much heat has flown from the gas on the right to the gas on the left?
FigureAns :
Page No 64: