NEET-XII-Physics
26: Laws of Thermodynamics
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #17Consider the cyclic process ABCA, shown in figure, performed on a sample of 2.0 mol of an ideal gas. A total of 1200 J of heat is withdrawn from the sample in the process. Find the work done by the gas during the part BC.
FigureAns : Given:
Number of moles of the gas, n = 2
∆Q = - 1200 J (Negative sign shows that heat is extracted out from the system)
∆U = 0 (During cyclic process)
Using the first law of thermodynamics, we get
∆Q = ∆U + ∆W
⇒ -1200 = 0 + (WAB + WBC + WCA)
Since the change in volume of the system applies on line CA, work done during CA will be zero.
From the ideal gas equation, we get
PV = nRT
P∆V = nR∆T
W = P∆V = nR∆T
⇒ ∆Q = ∆U + ∆W
⇒ -1200 = nR∆T + WBC + 0
⇒ -1200 = 2 × 8.3 × 200 + WBC
WBC = - 400 × 8.3 - 1200
= - 4520 J
Page No 63:
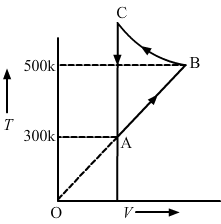
- Qstn #18Figure shows the variation in the internal energy U with the volume V of 2.0 mol of an ideal gas in a cyclic process abcda. The temperatures of the gas at b and c are 500 K and 300 K respectively. Calculate the heat absorbed by the gas during the process.
FigureAns : Given: Number of moles of the gas, n = 2 moles
The system's volume is constant for lines bc and da. Therefore,
∆V = 0
Thus, work done for paths da and bc is zero.
`` \Rightarrow {W}_{da}={W}_{bc}=0``
Since the process is cyclic, ∆U is equal to zero.
Using the first law, we get
∆W = ∆Q
∆W = ∆WAB + ∆WCD
Since the temperature is kept constant during lines ab and cd, these are isothermal expansions.
Work done during an isothermal process is given by
W = nRT`` \,\mathrm{\,ln\,}\frac{{V}_{f}}{{V}_{i}}``
If `` {V}_{f}\,\mathrm{\,and\,}{V}_{i}`` are the initial and final volumes during the isothermal process, then
`` W=n{\,\mathrm{\,RT\,}}_{1}ln\left(\frac{2{\,\mathrm{\,V\,}}_{0}}{{\,\mathrm{\,V\,}}_{0}}\right)+n{\,\mathrm{\,RT\,}}_{2}ln\left(\frac{{\,\mathrm{\,V\,}}_{0}}{2{\,\mathrm{\,V\,}}_{0}}\right)``
W = nR × 2.303 × log 2 × (500 - 300)
W = 2 × 8.314 × 2.303 × 0.301 × 200
W = 2305.31 J
Page No 63:
- Qstn #19Find the change in the internal energy of 2 kg of water as it is heated from 0°C to 4°C. The specific heat capacity of water is 4200 J kg-1 K-1 and its densities at 0°C and 4°C are 999.9 kg m-3 and 1000 kg m-3 respectively. Atmospheric pressure = 105 Pa.Ans : Given:
Mass of water, M = 2 kg
Change in temperature of the system, ∆θ = 4°C = 277 K
Specific heat of water, sw = 4200 J/kg-°C
Initial density, p0 = 999.9 kg/m3
Final density, pf = 1000 kg/m3
P = 105 Pa
Let change in internal energy be ∆U.
Using the first law of thermodynamics, we get
∆Q = ∆U + ∆W
Also, ∆Q = ms∆θ
W = P∆V = P(`` {V}_{f}-{V}_{i}``)
⇒ ms∆θ = ∆U + P (V0 - V4)
⇒ 2 × 4200 × 4= ∆U + 105 (∆V)
⇒ 33600 = ∆U + 105 `` \left(\frac{m}{{p}_{0}}-\frac{m}{{p}_{f}}\right)``
⇒ 33600 = ∆U + 105 × (`` -``0.0000002)
⇒ 33600 = ∆U `` -`` 0.02
∆U = (33600 `` -`` 0.02) J
Page No 63:
- Qstn #20Calculate the increase in the internal energy of 10 g of water when it is heated from 0°C to 100°C and converted into steam at 100 kPa. The density of steam = 0.6 kg m-3. Specific heat capacity of water = 4200 J kg-1 °C-1 and the latent heat of vaporization of water = 2.25 × 10 6J kg-1.Ans : Given:
Mass of water, m = 10 g = 0.01 kg
Pressure, P = 105 Pa
Specific heat capacity of water, c = 1000 J/Kg `` °C``
Latent heat, L = 2.25`` \times {10}^{6}\,\mathrm{\,J\,}/\,\mathrm{\,Kg\,}``
`` ∆t=\,\mathrm{\,Change\,}\,\mathrm{\,in\,}\,\mathrm{\,temperature\,}\,\mathrm{\,of\,}\,\mathrm{\,the\,}\,\mathrm{\,system\,}=100°\,\mathrm{\,C\,}=373\,\mathrm{\,K\,}``
∆Q = Heat absorbed to raise the temperature of water from 0`` °\,\mathrm{\,C\,}\,\mathrm{\,to\,}100°\,\mathrm{\,C\,}`` + Latent heat for conversion of water to steam
∆Q = `` mc∆t+mL``
= 0.01 × 4200 × 100 + 0.01 × 2.5 × 106
= 4200 + 25000 = 29200 J
∆W = P∆V
∆V`` =\,\mathrm{\,mass\,}\left(\frac{1}{\,\mathrm{\,final\,}\,\mathrm{\,density\,}}-\frac{1}{\,\mathrm{\,initial\,}\,\mathrm{\,density\,}}\right)``
`` ∆\,\mathrm{\,V\,}=\left(\frac{0.01}{0.6}\right)-\left(\frac{0.01}{1000}\right)=0.01699``
∆W = P∆V = 0.01699 × 105 = 1699 J
Using the first law, we get
∆Q = ∆W + ∆U
∆U = ∆Q - ∆W = 29200 - 1699
= 27501 = 2.75 × 104 J
Page No 64:
- Qstn #21Figure shows a cylindrical tube of volume V with adiabatic walls containing an ideal gas. The internal energy of this ideal gas is given by 1.5 nRT. The tube is divided into two equal parts by a fixed diathermic wall. Initially, the pressure and the temperature are p1, T1 on the left and p2, T2 on the right. The system is left for sufficient time so that the temperature becomes equal on the two sides.Ans :
Let n1 , U1 and n2 ,U2 be the no. of moles , internal energy of ideal gas in the left chamber and right chamber respectively.
- #21-aHow much work has been done by the gas on the left part?Ans : As the diathermic wall is fixed, so final volume of the chambers will be same. Thus, ΔV = 0, hence work done ΔW= PΔV = 0
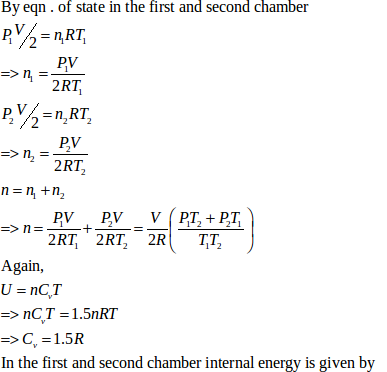
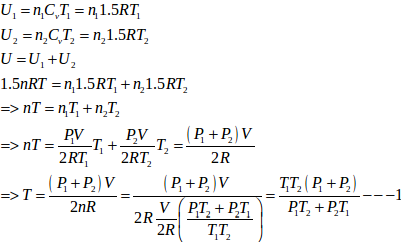
- #21-bFind the final pressures on the two sides.Ans : Let final pressure in the first and second compartment P1’ and P2’.
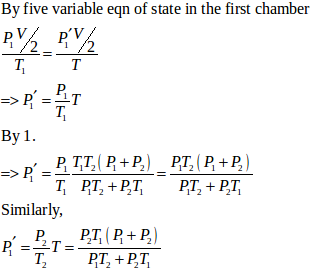
- #21-cFind the final equilibrium temperature.Ans :

- #21-dHow much heat has flown from the gas on the right to the gas on the left?
FigureAns :
Page No 64:
- Qstn #22An adiabatic vessel of total volume V is divided into two equal parts by a conducting separator. The separator is fixed in this position. The part on the left contains one mole of an ideal gas (U = 1.5 nRT) and the part on the right contains two moles of the same gas. Initially, the pressure on each side is p. The system is left for sufficient time so that a steady state is reached. Find
- #22-athe work done by the gas in the left part during the process,Ans : Since the conducting wall is fixed, the work done by the gas on the left part during the process is zero because the change in volume will be zero due to the fixed position of the wall.
- #22-bthe temperature on the two sides in the beginning,Ans : For left side:
Let the initial pressure on both sides of the wall be p.
We know,
Volume =`` \frac{\mathit{V}}{\mathit{2}}``
Number of moles, n = 1
Let initial temperature be T1.
Using the ideal gas equation, we get
`` \frac{\mathit{P}\mathit{V}}{\mathit{2}}\mathit{=}nR{T}_{\mathit{1}}``
`` \mathit{\Rightarrow }\frac{\mathit{P}\mathit{V}}{\mathit{2}}\mathit{=}\mathit{\left(}\mathit{1}\mathit{\right)}RT``
`` \mathit{\Rightarrow }{T}_{\mathit{1}}\mathit{=}\frac{\mathit{P}\mathit{V}}{\mathit{(}\mathit{2}\mathit{m}\mathit{o}\mathit{l}\mathit{e}\mathit{s}\mathit{)}\mathit{R}}``
For right side:
Number of moles, n = 2
Let the initial temperature be T2.
We know,
Volume =`` \frac{\mathit{V}}{\mathit{2}}``
`` \frac{\mathit{P}\mathit{V}}{\mathit{2}}\mathit{=}nR{T}_{\mathit{2}}``
`` \mathit{\Rightarrow }{T}_{\mathit{2}}\mathit{=}\frac{\mathit{P}\mathit{V}}{\mathit{(}\mathit{4}\mathit{m}\mathit{o}\mathit{l}\mathit{e}\mathit{s}\mathit{)}\mathit{R}}``
- #22-cthe final common temperature reached by the gases,Ans :
Here,
U = 1.5nRT
T = temperature at the equilibrium
P1 = P2 = P
n1 = 1 mol
n2 = 2 mol
Let T1 and T2 be the initial temperatures of the left and right chamber respectively.
Applying eqn. of state
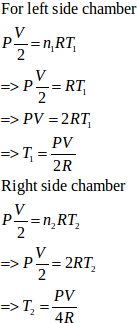
We know that total n = n1 + n2 = 3
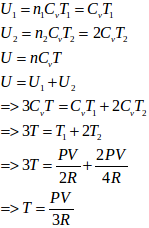
- #22-dthe heat given to the gas in the right part and
(d) the increase in the internal energy of the gas in the left part.Ans : For RHS:
∆Q = ∆U as ∆W = 0
∆U = 1.5 n2R (T - T2)
When T is the final temperature and T2 is the initial temperature of side 1, we get
= 1.5 × 2 × R (T - T2)
= 1.5 × 2 × `` \frac{4\,\mathrm{\,PV\,}-3\,\mathrm{\,PV\,}}{4\times 3\,\mathrm{\,mole\,}}``
`` ``
`` =\frac{3\times \,\mathrm{\,PV\,}}{4\times 3\,\mathrm{\,mole\,}}=\frac{\,\mathrm{\,PV\,}}{4}``
(d) If dW = 0, then using the first law, we get
dQ = - dU
⇒ dU = - dQ = `` \frac{-\,\mathrm{\,PV\,}}{4}``