NEET-XII-Physics
24: Kinetic Theory of Gases
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #12The quantity
pVkTrepresents
(a) mass of the gas
(b) kinetic energy of the gas
(c) number of moles of the gas
(d) number of molecules in the gas.digAnsr: dAns : `` \,\mathrm{\,Here\,},``
`` PV=nRT...\left(1\right)``
`` \,\mathrm{\,Also\,},``
`` k=\frac{R}{N}``
`` \Rightarrow R=kN...\left(2\right)``
`` ``
`` \,\mathrm{\,Now\,},``
`` PV=nkNT\left[\,\mathrm{\,From\,}\,\mathrm{\,eq\,}.\left(1\right)\,\mathrm{\,and\,}\,\mathrm{\,eq\,}.\left(2\right)\right]``
`` \Rightarrow nN=\frac{PV}{kT}``
`` nN=\,\mathrm{\,Number\,}\,\mathrm{\,of\,}\,\mathrm{\,molecules\,}``
`` \frac{\,\mathrm{\,P\,}\,\mathrm{\,V\,}}{\,\mathrm{\,k\,}\,\mathrm{\,T\,}}=\,\mathrm{\,Number\,}\,\mathrm{\,of\,}\,\mathrm{\,molecules\,}``
`` ``
`` ``
Thus,
(d) is the correct answer.
- Qstn #13The process on an ideal gas, shown in figure, is
(a) isothermal
(b) isobaric
(c) isochoric
(d) none of these.
Figure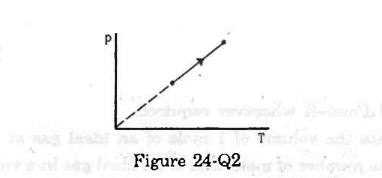 digAnsr: cAns : According to the graph, P is directly proportional to T.
digAnsr: cAns : According to the graph, P is directly proportional to T.
Applying the equation of state, we get
PV = nRT
`` \Rightarrow P=\frac{nR}{V}T``
`` ``
`` \,\mathrm{\,Given\,}:P\alpha T``
`` \,\mathrm{\,This\,}\,\mathrm{\,means\,}\frac{\,\mathrm{\,n\,}\,\mathrm{\,R\,}}{\,\mathrm{\,V\,}}\,\mathrm{\,is\,}\,\mathrm{\,a\,}\,\mathrm{\,constant\,}.\,\mathrm{\,So\,},\,\mathrm{\,V\,}\,\mathrm{\,is\,}\,\mathrm{\,also\,}\,\mathrm{\,a\,}\,\mathrm{\,constant\,}.``
Constant V implies the process is isochoric.
Thus,
(c) is the correct answer.
- Qstn #14There is some liquid in a closed bottle. The amount of liquid is continuously decreasing. The vapour in the remaining part
(a) must be saturated
(b) must be unsaturated
(c) may be saturated
(d) there will be no vapour.digAnsr: bAns : As the liquid is decreasing, the liquid is vapourised. We know that vapourisation cannot occur in saturated air and there cannot be any liquid with no vapour at all. So, the vapour in the remaining part is unsaturated.
Thus,
(b) is the correct answer.
- Qstn #15There is some liquid in a closed bottle. The amount of liquid remains constant as time passes. The vapour in the remaining part
(a) must be saturated
(b) must be unsaturated
(c) may be unsaturated
(d) there will be no vapour.digAnsr: aAns : Since the amount of liquid is constant, there is no vapourisation of the liquid inside the bottle. Also, since there cannot be a liquid with no vapours at all and vapourisation cannot take place in the remaining saturated part, the remaining part must be saturated with the vapours of the liquid.
Thus,
(a) is the correct answer.
- Qstn #16Vapour is injected at a uniform rate in a closed vessel which was initially evacuated. The pressure in the vessel
(a) increases continuously
(b) decreases continuously
(c) first increases and then decreases
(d) first increases and then becomes constant.digAnsr: dAns : As the vapour is injected, the pressure of the chamber increases. But when the pressure becomes equal to the saturated vapour pressure, it condenses. So, if more vapour is injected beyond the saturated vapour pressure, the vapour will condense and thus the vapour pressure will be constant.
Thus,
(d) is the correct answer.
- Qstn #17A vessel A has volume V and a vessel B has volume 2V. Both contain some water which has a constant volume. The pressure in the space above water is pa for vessel A and pb for vessel B.
(a) pa = pb
(b) pa = 2pb
(c) pb = 2pa
(d) pb = 4padigAnsr: aAns : The maximum pressure attainable above the water will be saturated vapour pressure at that temperature. Since saturated vapour pressure does not depend upon volume, both the vessels will have same pressure.
Thus,
(a) is the correct answer.
- #Section : iii
- Qstn #1Consider a collision between an oxygen molecule and a hydrogen molecule in a mixture of oxygen and hydrogen kept at room temperature. Which of the following are possible?
(a) The kinetic energies of both the molecules increase.
(b) The kinetic energies of both the molecules decrease.
(c) kinetic energy of the oxygen molecule increases and that of the hydrogen molecule decreases.
(d) The kinetic energy of the hydrogen molecule increases and that of the oxygen molecule decreases.digAnsr: c,dAns : According to Kinetic theory, postulates collision between molecules are elastic. This means that kinetic energy after any collision is conserved because while one one gains kinetic energy, another loses it. Both options,
(c) and
(d) consider the conservation of kinetic energy in the collision.
Thus,
(c) and
(d) are correct answers.
- Qstn #2Consider a mixture of oxygen and hydrogen kept at room temperature. As compared to a hydrogen molecule an oxygen molecule hits the wall
(a) with greater average speed
(b) with smaller average speed
(c) with greater average kinetic energy
(d) with smaller average kinetic energy.digAnsr: bAns : The average speed of molecules is given by `` \sqrt{\frac{8kT}{\pi m}}``. We observe that greater the mass, lesser is the average speed of the molecule. Since an oxygen molecule is heavier than a hydrogen molecule, the oxygen molecule will hit the wall with smaller average speed.
Thus,
(b) is the correct answer.
- Qstn #3Which of the following quantities is zero on an average for the molecules of an ideal gas in equilibrium?
(a) Kinetic energy
(b) Momentum
(c) Density
(d) Speed.digAnsr: bAns : The molecules move in all possible directions in an ideal gas at equilibrium. Since momentum is a vector quantity for every direction of motion of the molecules, there exists an opposite direction of motion of the other. Hence, the average momentum is zero for an ideal gas at equilibrium.
Thus,
(b) is the correct answer.
- Qstn #4Keeping the number of moles, volume and temperature the same, which of the following are the same for all ideal gases?
(a) Rms speed of a molecule
(b) Density
(c) Pressure
(d) Average magnitude of momentum.digAnsr: cAns : Pressure of an ideal gas is given by PV = `` \frac{1}{3}mn{u}^{2}``. We know that pressure depends on volume, number of molecules and root mean square velocity. Also, root mean square velocity depends on the temperature of the gas. Since the number of molecules, volume and temperature are constant, pressure of the gas will not change.
Thus,
(c) is the correct answer.
- Qstn #5The average momentum of a molecule in a sample of an ideal gas depends on
(a) temperature
(b) number of moles
(c) volume
(d) none of these.digAnsr: dAns : Average momentum of a gas sample is zero, so it does not depend upon any of these parameters.
Thus,
(d) is the correct answer.
- Qstn #6Which of the following quantities is the same for all ideal gases at the same temperature?
(a) The kinetic energy of 1 mole
(b) The kinetic energy of 1 g
(c) The number of molecules in 1 mole
(d) The number of molecules in 1 gdigAnsr: a,cAns : (a) The kinetic energy of 1 mole
(c) The number of molecules in 1 mole
Kinetic energy per mole of an ideal gas is directly proportional to T. So, it will be the same for all ideal gases.
Number of molecules in 1 mole of an ideal is the same for all ideal gases because ideal gases obey Avogadro's law.
Thus,
(a) and
(c) are correct answers.
- Qstn #7Consider the quantity
MkTpVof an ideal gas where M is the mass of the gas. It depends on the
(a) temperature of the gas
(b) volume of the gas
(c) pressure of the gas
(d) nature of the gas.digAnsr: dAns : `` \,\mathrm{\,In\,}\,\mathrm{\,an\,}\,\mathrm{\,ideal\,}\,\mathrm{\,gas\,},\,\mathrm{\,the\,}\,\mathrm{\,equation\,}\,\mathrm{\,of\,}\,\mathrm{\,state\,}\,\mathrm{\,is\,}\,\mathrm{\,given\,}\,\mathrm{\,by\,}``
`` PV=nRT``
`` \Rightarrow PV=n{N}_{A}\frac{R}{{N}_{A}}T``
`` \Rightarrow PV=n{N}_{A}kT``
`` \Rightarrow \frac{1}{n{N}_{A}}=\frac{kT}{PV}``
`` ``
`` \,\mathrm{\,Multiplying\,}\,\mathrm{\,both\,}\,\mathrm{\,sides\,}\,\mathrm{\,by\,}\,\mathrm{\,mass\,}\,\mathrm{\,of\,}\,\mathrm{\,the\,}\,\mathrm{\,gas\,}\,\mathrm{\,M\,},\,\mathrm{\,we\,}\,\mathrm{\,get\,}``
`` \frac{M}{n{N}_{A}}=\frac{MkT}{PV}``
`` \,\mathrm{\,Now\,},n{N}_{A}\,\mathrm{\,gives\,}\,\mathrm{\,the\,}\,\mathrm{\,total\,}\,\mathrm{\,number\,}\,\mathrm{\,of\,}\,\mathrm{\,molecules\,}\,\mathrm{\,of\,}\,\mathrm{\,the\,}\,\mathrm{\,gas\,}.``
`` \,\mathrm{\,Also\,},\frac{M}{n{N}_{A}}\,\mathrm{\,gives\,}\,\mathrm{\,the\,}\,\mathrm{\,mass\,}\,\mathrm{\,of\,}\,\mathrm{\,a\,}\,\mathrm{\,single\,}\,\mathrm{\,molecule\,}.``
`` \,\mathrm{\,Hence\,},\frac{\,\mathrm{\,M\,}\,\mathrm{\,k\,}\,\mathrm{\,T\,}}{\,\mathrm{\,P\,}\,\mathrm{\,V\,}}\,\mathrm{\,is\,}\,\mathrm{\,the\,}\,\mathrm{\,mass\,}\,\mathrm{\,of\,}\,\mathrm{\,a\,}\,\mathrm{\,single\,}\,\mathrm{\,molecule\,}\,\mathrm{\,of\,}\,\mathrm{\,the\,}\,\mathrm{\,gas\,},``
`` \,\mathrm{\,Molecular\,}\,\mathrm{\,mass\,}\,\mathrm{\,is\,}\,\mathrm{\,a\,}\,\mathrm{\,property\,}\,\mathrm{\,of\,}\,\mathrm{\,the\,}\,\mathrm{\,gas\,}.``
`` ``
Thus,
(d) is the correct answer.
- #Section : iv