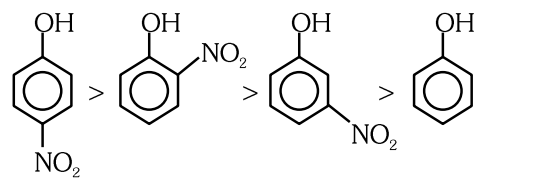
Given below are two statements : Statement I :The acidic strength of monosubstituted nitrophenol is higher than phenol because of electron withdrawing nitro group. Statement II : `` o `` -nitrophenol, `` m `` -nitrophenol and `` p `` -nitrophenol will have same acidic strength as they have one nitro group attached to the phenolic ring. In the light of the above statements, choose the most appropriate answer from the options given below :
(A)Statement I is incorrect but Statement II is correct.
(B)Both Statement I and Statement II are correct.
(C)Both Statement I and Statement II are incorrect.
(D)Statement I is correct but Statement II is incorrect.