NEET-XII-Chemistry
c2022 year:2022
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #NEET 2022 Chemistry Questions with
Answer s Key Solutions
- Qstn #1The `` pH `` of the solution containing `` 50\, mL `` each of `` 0.10 \,M `` sodium acetate and `` 0.01 \,M `` acetic acid is [Given `` pK _{ a } `` of `` \left.CH _{3} COOH =4.57\right] ``
(A) `` 2.57 ``
(B) `` 5.57 ``
(C) `` 3.57 ``
(D) `` 4.57 ``digAnsr: BAns : Weak acid `` \left( CH _{3} COOH \right) `` and salt of weak acid-strong base `` \left( CH _{3} COONa \right) `` form an acidic buffer.
Sodium acetate `` \left( CH _{3} COONa \right)=0.10 \,M `` ;
Acetic acid `` \left( CH _{3} COOH \right)=0.01 \,M `` ;
`` pH `` of acidic buffer solution is given by
`` pH = pK _{ a }+\log \frac{[\text { Salt }]}{[\text { Acid }]} ``
`` =4.57+\log \left(\frac{0.1}{0.01}\right) ``
`` =5.57 ``
- Qstn #2Which one is not correct mathematical equation for Dalton's Law of partial pressure ? Here `` p= `` total pressure of gaseous mixture
(A) `` p _{i}=\chi_{i} p _{i}^{0} `` , where `` \chi_{i}= `` mole fraction of `` i^{\text {th }} `` gas in gaseous mixture `` p_{i}^{0}= `` pressure of `` i^{\text {th }} `` gas in pure state
(B) `` p = p _{1}+ p _{2}+ p _{3} ``
(C) `` p = n _{1} \frac{ RT }{ V }+ n _{2} \frac{ RT }{ V }+ n _{3} \frac{ RT }{ V } ``
(D) `` p _{i}=\chi_{i} p `` , where `` p _{j}= `` partial pressure of `` i^{\text {th }} `` gas `` \chi_{i}= `` mole fraction of `` i^{\text {th }} `` gas in gaseous mixturedigAnsr: AAns : Dalton's law of partial pressure :
Partial pressure of gas `` = `` mole fraction of gas in gaseous mixture `` \times `` Total pressure of gaseous mixture.
`` p _{1} = X _{1} p ``
`` p _{2} = X _{2} p ``
`` p _{3} = X _{3} p ``
Total pressure,
`` p = p _{1}+ p _{2}+ p _{3} ``
- Qstn #3The incorrect statement regarding enzymes is :
(A)Enzymes are very specific for a particular reaction and substrate.
(B)Enzymes are biocatalysts.
(C)Like chemical catalysts enzymes reduce the activation energy of bio processes.
(D)Enzymes are polysaccharides.digAnsr: DAns : Which is incorrect statement regarding enzymes
(1) Like chemical catalysts enzymes reduce the activation energy of bio process `` \Rightarrow `` This is correct statement.
(2) Enzymes are polysaccharides `` \Rightarrow `` This is incorrect statement because enzymes are protein in nature
(3) Enzymes are very specific for a particular reaction and substrate `` \Rightarrow `` This is correct statement.
(4) Enzymes are biocatalyst `` \Rightarrow `` This is correct statement.
- Qstn #4Match List - I with List - II.
Choose the correct answer from the options given below:List I List II a Li i Absorbent for carbon dioxide b Na ii electrochemical cells c KOH iii Coolant in fast breeder reactors d Cs iv photoelectric cell
(A)
(a) - (ii),
(b) - (iii),
(c) - (i),
(d) - (iv)
(B)
(a) - (iv),
(b) - (i),
(c) - (iii),
(d) - (ii)
(C)
(a) - (iii),
(b) - (iv),
(c) - (ii),
(d) - (i)
(D)
(a) - (i),
(b) - (iii),
(c) - (iv),
(d) - (ii)digAnsr: AAns : `` Li `` - Electrochemical cells
`` Na `` - Coolant in fast breeder reactors
`` KOH `` - absorbent for `` CO _{2} ``
`` Cs `` - Photoelectric cell.
- Qstn #5Given below are two statements: Statement I : Primary aliphatic amines react with `` HNO _{2} `` to give unstable diazonium salts. Statement II : Primary aromatic amines react with `` HNO _{2} `` to form diazonium salts which are stable even above `` 300\, K `` . In the light of the above statements, choose the most appropriate answer from the options given below :
(A)Statement I is incorrect but Statement II is correct.
(B)Both Statement I and Statement II are correct.
(C)Both Statement I and Statement II are incorrect.
(D)Statement I is correct but Statement II is incorrect.digAnsr: DAns :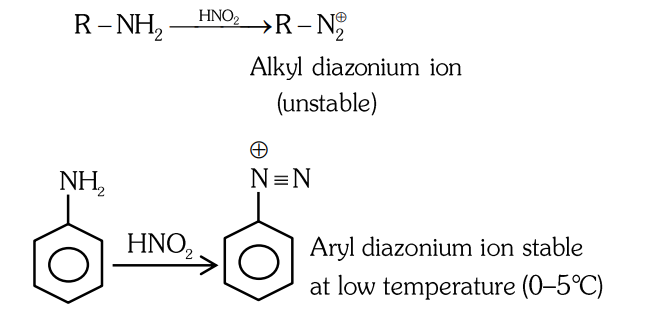
- Qstn #6Which of the following is suitable to synthesize chlorobenzene?
(A)
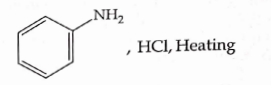
(B)Benzene, `` Cl _{2} `` , anhydrous `` FeCl _{3} ``
(C)Phenol, `` NaNO _{2}, HCl , CuCl ``
(D)
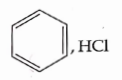 digAnsr: CAns :
digAnsr: CAns :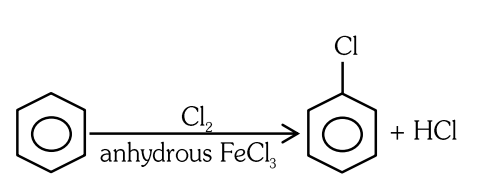
- Qstn #7Identify the incorrect statement from the following
(A)Lithium is the strongest reducing agentamong the alkali metals.
(B)Alkali metals react with water to form their hydroxides.
(C)The oxidation number of `` K `` in `` KO _{2} `` is `` +4 `` .
(D)Ionisation enthalpy of alkali metals decreases from top to bottom in the group.digAnsr: CAns : `` KO _{2} ``
`` K ^{+} O _{2}^{-}\left( O _{2}^{-}-\right. `` superoxide ion `` ) ``
- Qstn #8Which compound amongst the following is not an aromatic compound?
(A)
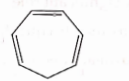
(B)
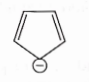
(C)
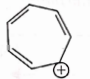
(D)
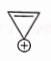 digAnsr: AAns :
digAnsr: AAns :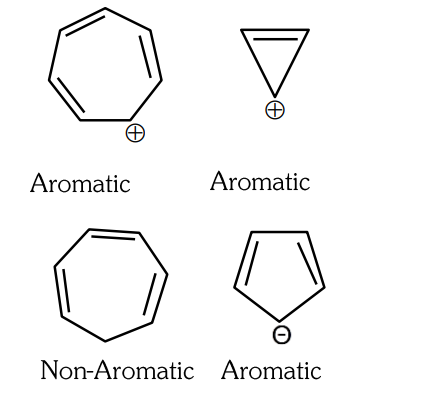
- Qstn #9At `` 298\, K `` , the standard electrode potentials of `` Cu ^{2+} /Cu , Zn ^{2+} / Zn , Fe ^{2+} / Fe `` and `` Ag ^{+} / Ag `` are `` 0.34\, V `` , `` -0.76 \,V ,-0.44 \,V `` and `` 0.80 \,V `` , respectively. On the basis of standard electrode potential, predict which of the following reaction can not occur?
(A) `` 2 CuSO _{4}( aq )+2 Ag ( s ) \rightarrow 2 Cu ( s )+ Ag _{2} SO _{4}( aq ) ``
(B) `` CuSO _{4}( aq )+ Zn ( s ) \rightarrow ZnSO _{4}( aq )+ Cu ( s ) ``
(C) `` CuSO _{4}( aq )+ Fe ( s ) \rightarrow FeSO _{4}( aq )+ Cu ( s ) ``
(D) `` FeSO _{4}( aq )+ Zn ( s ) \rightarrow ZnSO _{4}( aq )+ Fe ( s ) ``digAnsr: AAns : SRP : `` E _{ Zn ^{2+} / Zn }^{\circ}< E _{ Fe ^{2+} / Fe }^{\circ}< E _{ Cu ^{2+} / Cu }^{\circ}< E _{ Ag ^{+} / Ag }^{\circ} ``
Reactivity order : `` Zn > Fe > Cu > Ag ``
In case of displacement reaction, more reactive metals (lower SRP) can displace less reactive metals (higher SRP) from their salt solution.
`` CuSO _{4(\text { aq. })}+2 Ag _{( s )} \rightarrow Cu _{( s )}+ Ag _{2} SO _{4(\text { aq. })} ``
Option (3)
Reaction is not possible
as `` Ag `` is less reactive metal compare to `` Cu `` .
- Qstn #10Given below are two statements : Statement I :The acidic strength of monosubstituted nitrophenol is higher than phenol because of electron withdrawing nitro group. Statement II : `` o `` -nitrophenol, `` m `` -nitrophenol and `` p `` -nitrophenol will have same acidic strength as they have one nitro group attached to the phenolic ring. In the light of the above statements, choose the most appropriate answer from the options given below :
(A)Statement I is incorrect but Statement II is correct.
(B)Both Statement I and Statement II are correct.
(C)Both Statement I and Statement II are incorrect.
(D)Statement I is correct but Statement II is incorrect.digAnsr: AAns : Acidic strength of phenolic group increases due to electron withdrawing groups.
Order of acidic strength
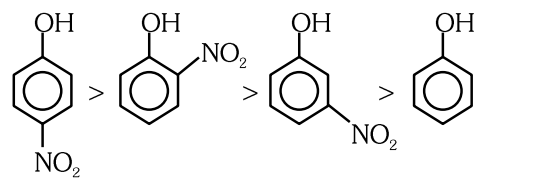
- Qstn #11Which of the following statement is not correct about diborane?
(A)Both the Boron atoms are `` s p^{2} `` hybridised.
(B)There are two 3-centre-2-electron bonds.
(C)The four terminal B-H bonds are two centre two electron bonds.
(D)The four terminal Hydrogen atoms and the two Boron atoms lie in one plane.digAnsr: AAns :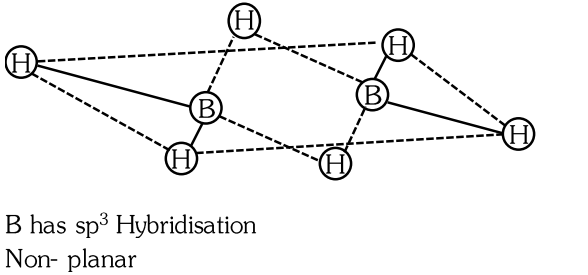
- Qstn #12Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R). Assertion (A) : In a particular point defect, an ionic solid is electrically neutral, even if few of its cations are missing from its unit cells. Reason (R) : In an ionic solid, Frenkel defect arises due to dislocation of cation from its lattice site to interstitial site, maintaining overall electrical neutrality. In the light of the above statements, choose the most appropriate answer from the options given below :
(A)(A) is not correct but (R) is correct
(B)Both (A) and (R) are correct and (R) is the correct explanation of (A)
(C)Both (A) and (R) are correct but (R) is not the correct explanation of `` ( A ) ``
(D)(A) is correct but (R) is not correctdigAnsr: BAns : (i) Statement-1 is correct because in point defects of ionic solid electrical neutrality is essential condition (given question is example of metal deficiency defect)
(ii) Statement-2 is correct because In Frenkel defect cation dislocate from lattice site to interstitial position.
(iii) Both statement are correct but statement-2 is not correct explanation of statement-1
- Qstn #13Given below are two statements : Statement I : The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions. Statement II : The boiling points of aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding. In the light of the above statements, choose the most appropriate answer from the options given below :
(A)Statement I is incorrect but Statement II is correct.
(B)Both Statement I and Statement II are correct.
(C)Both Statement I and Statement II are incorrect.
(D)Statement I is correct but Statement II is incorrect.digAnsr: BAns : Boiling point of comparable molecular mass molecules

- Qstn #14Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R). Assertion (A): `` ICl `` is more reactive than `` I _{2} `` . Reason (R): `` I-Cl `` bond is weaker than `` I-I `` bond. In the light of the above statements, choose the most appropriate answer from the options given below :
(A)(A) is not correct but (R) is correct.
(B)Both (A) and (R) are correct and (R) is the correct explanation of `` ( A ) `` .
(C)Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(D)(A) is correct but (R) is not correct.digAnsr: BAns : Interhalogen compound group `` 17^{\text {th }} ``
`` ICl `` is more reactive due to polar bonds.
From NCERT - X-X' bond is weaker than `` X - X `` bond except `` F _{2} ``