NEET-XII-Chemistry
Previous Year Paper year:2020
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #91Identify a molecule which does not exist.
(1) ``\ce{He2}``
(2) ``\ce{Li2}``
(3) ``\ce{C2}``
(4) ``\ce{O2}``digAnsr: 1Ans : 1
- Qstn #92Find out the solubility of ``\ce{Ni(OH)2}``
in 0.1 M NaOH.
Given that the ionic product of ``\ce{Ni(OH)2}``
is
2×``10^{-15}``.
(1) 2×``10^{-13}`` M
(2) 2×``10^{-8}`` M
(3) 1×``10^{-13}`` M
(4) 1×``10^{8}`` MdigAnsr: 1Ans : 1
- Qstn #93Identify the correct statements from the
following :
-(a) C``\ce{O2}``
(g) is used as refrigerant for ice-cream
and frozen food.
(b) The structure of C
60
contains twelve six
carbon rings and twenty five carbon rings.
(c) ZSM-5, a type of zeolite, is used to convert
alcohols into gasoline.
(d) CO is colorless and odourless gas.
(1) -(a), (b) and (c) only
(2) -(a) and (c) only
(3) (b) and (c) only
(4) (c) and (d) onlydigAnsr: 4Ans : 4
- Qstn #94Hydrolysis of sucrose is given by the following
reaction.
Sucrose+H2O ``<=>`` Glucose+Fructose
If the equilibrium constant (Kc) is 2×1013 at 300 K, the value of ∆rGs at the same temperature
will be :
(1) -8.314 J mol-1K-1×300 K×ln(2×1013)
(2) 8.314 J mol-1K-1×300 K×ln(2×1013)
(3) 8.314 J mol-1K-1×300 K×ln(3×1013)
(4) -8.314 J mol-1K-1×300 K×ln(4×1013)digAnsr: 1Ans : 1
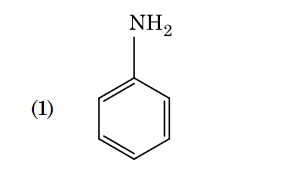
- Qstn #95Identify compound X in the following sequence of
reactions :

(1)

(2)
(3)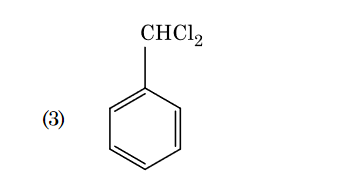
(4)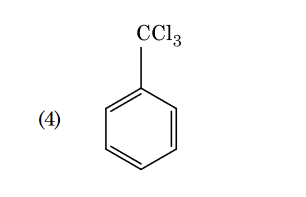 digAnsr: 3Ans : 3
digAnsr: 3Ans : 3
- Qstn #96Identify the incorrect match.
Name IUPAC Official Name
-(a) Unnilunium (i) Mendelevium
(b) Unniltrium (ii) Lawrencium
(c) Unnilhexium (iii) Seaborgium
(d) Unununnium (iv) Darmstadtium
(1) -(a), (i)
(2) (b), (ii)
(3) (c), (iii)
(4) (d), (iv)digAnsr: 4Ans : 4
- Qstn #97An element has a body centered cubic (bcc)
structure with a cell edge of 288 pm. The atomic
radius is :
(1) ``\frac{\sqrt3}{4} \times 288`` pm
(2) ``\frac{\sqrt2}{4} \times 288`` pm
(3) ``\frac{\sqrt4}{3} \times 288`` pm
(4) ``\frac{4}{\sqrt2}\times 288`` pmdigAnsr: 1Ans : 1
- Qstn #98Which of the following set of molecules will have
zero dipole moment ?
(1) Ammonia, beryllium difluoride, water,
1,4-dichlorobenzene
(2) Boron trifluoride, hydrogen fluoride, carbon
dioxide, 1,3-dichlorobenzene
(3) Nitrogen trifluoride, beryllium difluoride,
water, 1,3-dichlorobenzene
(4) Boron trifluoride, beryllium difluoride,
carbon dioxide, 1,4-dichlorobenzenedigAnsr: 4Ans : 4
- Qstn #99On electrolysis of dil.sulphuric acid using
Platinum (Pt) electrode, the product obtained at
anode will be :
(1) Hydrogen gas
(2) Oxygen gas
(3) H2S gas
(4) ``\ce{SO2}`` gasdigAnsr: 2Ans : 2
- Qstn #100Reaction between acetone and methylmagnesium
chloride followed by hydrolysis will give :
(1) Isopropyl alcohol
(2) Sec. butyl alcohol
(3) Tert. butyl alcohol
(4) Isobutyl alcoholdigAnsr: 3Ans : 3
- Qstn #101Which of the following oxoacid of sulphur has
-O-O- linkage ?
(1) ``\ce{H2SO3}``
, sulphurous acid
(2) ``\ce{H2SO4}``
, sulphuric acid
(3) ``\ce{H2S2O8}``
, peroxodisulphuric acid
(4) ``\ce{H2S2O7}``
, pyrosulphuric aciddigAnsr: 3Ans : 3
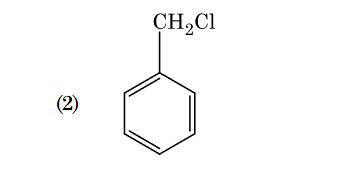
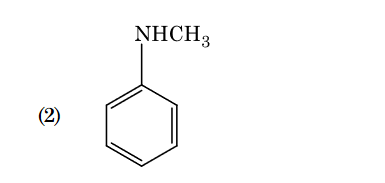
- Qstn #102Which of the following amine will give the
carbylamine test ?
(1)
(2)
(3)
(4)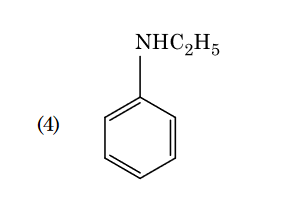 digAnsr: 1Ans : 1
digAnsr: 1Ans : 1
- Qstn #103The calculated spin only magnetic moment of Cr2+
ion is :
(1) 3.87 BM
(2) 4.90 BM
(3) 5.92 BM
(4) 2.84 BMdigAnsr: 2Ans : 2
- Qstn #104The correct option for free expansion of an ideal
gas under adiabatic condition is :
(1) q=0, ∆T=0 and w=0
(2) q=0, ∆T < 0 and w > 0
(3) q < 0, ∆T=0 and w=0
(4) q > 0, ∆T > 0 and w > 0digAnsr: 1Ans : 1