NEET-XII-Physics
13: Nuclei
- #12Find the Q-value and the kinetic energy of the emitted α-particle in the α-decay of (a)
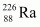 and (b)
and (b) 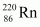 .
.
Given
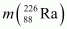 = 226.02540 u,
= 226.02540 u, 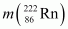 = 222.01750 u,
= 222.01750 u,
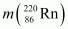 = 220.01137 u,
= 220.01137 u, 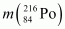 = 216.00189 u.
= 216.00189 u.
Ans : null (a) Alpha particle decay of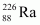 emits a helium nucleus. As a result, its mass number reduces to (226 - 4) 222 and its atomic number reduces to (88 - 2) 86. This is shown in the following nuclear reaction.
emits a helium nucleus. As a result, its mass number reduces to (226 - 4) 222 and its atomic number reduces to (88 - 2) 86. This is shown in the following nuclear reaction.
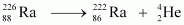
Q-value of
emitted α-particle = (Sum of initial mass - Sum of final mass) c2
Where,
c = Speed of light
It is given that:
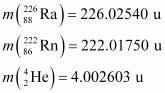
Q-value = [226.02540 - (222.01750 + 4.002603)] u c2
= 0.005297 u c2
But 1 u = 931.5 MeV/c2
∴Q = 0.005297 × 931.5 ≈ 4.94 MeV
Kinetic energy of the α-particle
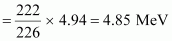
(b) Alpha particle decay of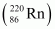 is shown by the following nuclear reaction.
is shown by the following nuclear reaction.
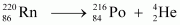
It is given that:
Mass of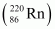 = 220.01137 u
= 220.01137 u
Mass of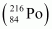 = 216.00189 u
= 216.00189 u
∴Q-value =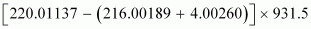
≈ 641 MeV
Kinetic energy of the α-particle
= 6.29 MeV
- #12-a
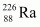 andAns : Alpha particle decay of
andAns : Alpha particle decay of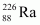 emits a helium nucleus. As a result, its mass number reduces to (226 - 4) 222 and its atomic number reduces to (88 - 2) 86. This is shown in the following nuclear reaction.
emits a helium nucleus. As a result, its mass number reduces to (226 - 4) 222 and its atomic number reduces to (88 - 2) 86. This is shown in the following nuclear reaction.
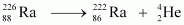
Q-value of
emitted α-particle = (Sum of initial mass - Sum of final mass) c2
Where,
c = Speed of light
It is given that:
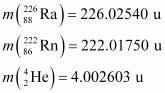
Q-value = [226.02540 - (222.01750 + 4.002603)] u c2
= 0.005297 u c2
But 1 u = 931.5 MeV/c2
∴Q = 0.005297 × 931.5 ≈ 4.94 MeV
Kinetic energy of the α-particle
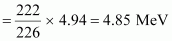
- #12-b
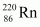 .
.
Given
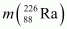 = 226.02540 u,
= 226.02540 u, 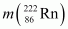 = 222.01750 u,
= 222.01750 u,
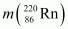 = 220.01137 u,
= 220.01137 u, 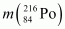 = 216.00189 u.
= 216.00189 u.
Ans : Alpha particle decay of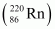 is shown by the following nuclear reaction.
is shown by the following nuclear reaction.
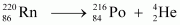
It is given that:
Mass of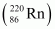 = 220.01137 u
= 220.01137 u
Mass of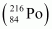 = 216.00189 u
= 216.00189 u
∴Q-value =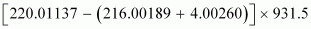
≈ 641 MeV
Kinetic energy of the α-particle
= 6.29 MeV