NEET-XII-Physics
13: Nuclei
- #10The half-life of
 is 28 years. What is the disintegration rate of 15 mg of this isotope?
is 28 years. What is the disintegration rate of 15 mg of this isotope?
Ans : Half life of ,
, 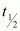 = 28 years
= 28 years
= 28 × 365 × 24 × 60 × 60
= 8.83 × 108 s
Mass of the isotope, m = 15 mg
90 g of atom contains 6.023 × 1023 (Avogadro’s number) atoms.
atom contains 6.023 × 1023 (Avogadro’s number) atoms.
Therefore, 15 mg of contains:
contains:

Rate of disintegration,.gif)
Where,
λ = Decay constant
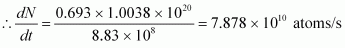
Hence, the disintegration rate of 15 mg of the given isotope is 7.878 × 1010 atoms/s.