NEET-XII-Physics
13: Nuclei
- #7A radioactive isotope has a half-life of T years. How long will it take the activity to reduce to
a) 3.125%, b) 1% of its original value?
Ans : Half-life of the radioactive isotope = T years
Original amount of the radioactive isotope = N0
a) After decay, the amount of the radioactive isotope = N
It is given that only 3.125% of N0 remains after decay. Hence, we can write:

Where,
λ = Decay constant
t = Time
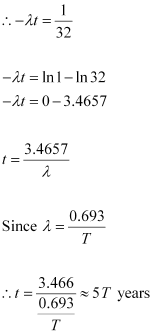
Hence, the isotope will take about 5T years to reduce to 3.125% of its original value.
b) After decay, the amount of the radioactive isotope = N
It is given that only 1% of N0 remains after decay. Hence, we can write:
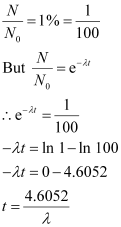
Since, λ = 0.693/T
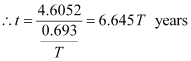
Hence, the isotope will take about 6.645T years to reduce to 1% of its original value.