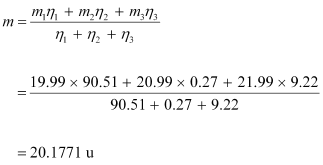NEET-XII-Physics
13: Nuclei
- #2The three stable isotopes of neon:
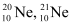 and
and
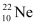 have respective abundances of 90.51%, 0.27% and 9.22%. The atomic masses of the three isotopes are 19.99 u, 20.99 u and 21.99 u, respectively. Obtain the average atomic mass of neon.
have respective abundances of 90.51%, 0.27% and 9.22%. The atomic masses of the three isotopes are 19.99 u, 20.99 u and 21.99 u, respectively. Obtain the average atomic mass of neon.
Ans : Atomic mass of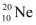 , m1= 19.99 u
, m1= 19.99 u
Abundance of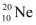 , η1 = 90.51%
, η1 = 90.51%
Atomic mass of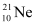 , m2 = 20.99 u
, m2 = 20.99 u
Abundance of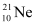 , η2 = 0.27%
, η2 = 0.27%
Atomic mass of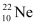 , m3 = 21.99 u
, m3 = 21.99 u
Abundance of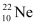 , η3 = 9.22%
, η3 = 9.22%
The average atomic mass of neon is given as: