NEET-XII-Physics
12: Atoms
- #8The radius of the innermost electron orbit of a hydrogen atom is 5.3 ×10-11 m. What are the radii of the n = 2 and n =3 orbits?
Ans : The radius of the innermost orbit of a hydrogen atom, r1 = 5.3 × 10-11 m.
Let r2 be the radius of the orbit at n = 2. It is related to the radius of the innermost orbit as:

For n = 3, we can write the corresponding electron radius as:
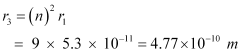
Hence, the radii of an electron for n = 2 and n = 3 orbits are 2.12 × 10-10 m and 4.77 × 10-10 m respectively.