Loading…
NEET-XII-Physics
11: Dual Nature Of Radiation And Matter
- #26Ultraviolet light of wavelength 2271 Å from a 100 W mercury source irradiates a photo-cell made of molybdenum metal. If the stopping potential is -1.3 V, estimate the work function of the metal. How would the photo-cell respond to a high intensity (∼105 W m-2) red light of wavelength 6328 Å produced by a He-Ne laser?
Ans : Wavelength of ultraviolet light, λ = 2271 Å = 2271 × 10-10 m
Stopping potential of the metal, V0 = 1.3 V
Planck’s constant, h = 6.6 × 10-34 J
Charge on an electron, e = 1.6 × 10-19 C
Work function of the metal =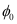
Frequency of light = ν
We have the photo-energy relation from the photoelectric effect as:
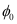 = hν - eV0
= hν - eV0
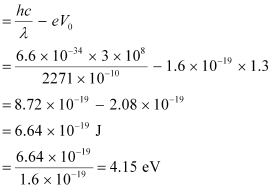
Let ν0 be the threshold frequency of the metal.
∴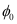 = hν0
= hν0
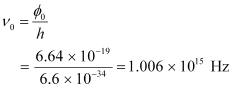
Wavelength of red light, = 6328 × 10-10 m
= 6328 × 10-10 m
∴Frequency of red light,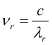
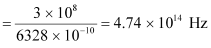
Since ν0> νr, the photocell will not respond to the red light produced by the laser.