NEET-XII-Physics
11: Dual Nature Of Radiation And Matter
- #19What is the de Broglie wavelength of a nitrogen molecule in air at 300 K? Assume that the molecule is moving with the root-mean square speed of molecules at this temperature. (Atomic mass of nitrogen = 14.0076 u)
Ans : Temperature of the nitrogen molecule, T = 300 K
Atomic mass of nitrogen = 14.0076 u
Hence, mass of the nitrogen molecule, m = 2 × 14.0076 = 28.0152 u
But 1 u = 1.66 × 10-27 kg
∴m = 28.0152 ×1.66 × 10-27 kg
Planck’s constant, h = 6.63 × 10-34 Js
Boltzmann constant, k = 1.38 × 10-23 J K-1
We have the expression that relates mean kinetic energy of the nitrogen molecule with the root mean square speed
of the nitrogen molecule with the root mean square speed  as:
as:
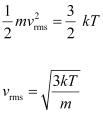
Hence, the de Broglie wavelength of the nitrogen molecule is given as:
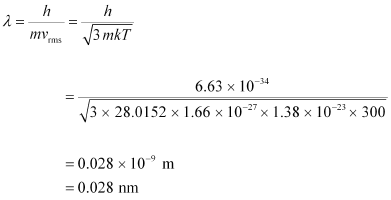
Therefore, the de Broglie wavelength of the nitrogen molecule is 0.028 nm.