NEET-XII-Physics
01: Electric Charges And Fields
- #11A polythene piece rubbed with wool is found to have a negative charge of 3 × 10-7 C.
(a) Estimate the number of electrons transferred (from which to which?)
(b) Is there a transfer of mass from wool to polythene?
(a) Estimate the number of electrons transferred (from which to which?)
(b) Is there a transfer of mass from wool to polythene?Ans : null (a) When polythene is rubbed against wool, a number of electrons get transferred from wool to polythene. Hence, wool becomes positively charged and polythene becomes negatively charged.
Amount of charge on the polythene piece, q = -3 × 10-7 C
Amount of charge on an electron, e = -1.6 × 10-19 C
Number of electrons transferred from wool to polythene = n
n can be calculated using the relation,
q = ne
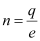
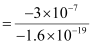
= 1.87 × 1012
Therefore, the number of electrons transferred from wool to polythene is 1.87 × 1012.
(b) Yes.
There is a transfer of mass taking place. This is because an electron has mass,
me = 9.1 × 10-3 kg
Total mass transferred to polythene from wool,
m = me × n
= 9.1 × 10-31 × 1.85 × 1012
= 1.706 × 10-18 kg
Hence, a negligible amount of mass is transferred from wool to polythene.
(a) When polythene is rubbed against wool, a number of electrons get transferred from wool to polythene. Hence, wool becomes positively charged and polythene becomes negatively charged.
Amount of charge on the polythene piece, q = -3 × 10-7 C
Amount of charge on an electron, e = -1.6 × 10-19 C
Number of electrons transferred from wool to polythene = n
n can be calculated using the relation,
q = ne
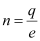
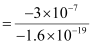
= 1.87 × 1012
Therefore, the number of electrons transferred from wool to polythene is 1.87 × 1012.
(b) Yes.
There is a transfer of mass taking place. This is because an electron has mass,
me = 9.1 × 10-3 kg
Total mass transferred to polythene from wool,
m = me × n
= 9.1 × 10-31 × 1.85 × 1012
= 1.706 × 10-18 kg
Hence, a negligible amount of mass is transferred from wool to polythene.
- #11-aEstimate the number of electrons transferred (from which to which?)Ans : When polythene is rubbed against wool, a number of electrons get transferred from wool to polythene. Hence, wool becomes positively charged and polythene becomes negatively charged.
Amount of charge on the polythene piece, q = -3 × 10-7 C
Amount of charge on an electron, e = -1.6 × 10-19 C
Number of electrons transferred from wool to polythene = n
n can be calculated using the relation,
q = ne
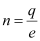
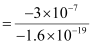
= 1.87 × 1012
Therefore, the number of electrons transferred from wool to polythene is 1.87 × 1012.
- #11-bIs there a transfer of mass from wool to polythene?Ans : Yes.
There is a transfer of mass taking place. This is because an electron has mass,
me = 9.1 × 10-3 kg
Total mass transferred to polythene from wool,
m = me × n
= 9.1 × 10-31 × 1.85 × 1012
= 1.706 × 10-18 kg
Hence, a negligible amount of mass is transferred from wool to polythene.