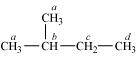NEET-XII-Chemistry
01: Haloalkanes and Haloarenes
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #1 - Haloalkanes and Haloarenes
- #Section : ISECTION I Page No 285:
- Qstn #2Why is sulphuric acid not used during the reaction of alcohols with KI?
Ans : In the presence of sulphuric acid (H2SO4), KI produces HI

Since
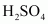 is an oxidizing agent, it oxidizes HI (produced in the reaction to I2).
is an oxidizing agent, it oxidizes HI (produced in the reaction to I2).

As a result, the reaction between alcohol and HI to produce alkyl iodide cannot occur. Therefore, sulphuric acid is not used during the reaction of alcohols with KI. Instead, a non-oxidizing acid such as H3PO4 is used.
- Qstn #3Write structures of different dihalogen derivatives of propane.
Ans : There are four different dihalogen derivatives of propane. The structures of these derivatives are shown below.
(i)
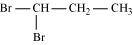
1, 1-Dibromopropane
(ii)
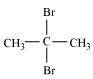
2, 2-Dibromopropane
(iii)
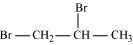
1, 2-Dibromopropane
(iv)

1, 3-Dibromopropane
- Qstn #4Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields
- Qstn #4-iA single monochloride.Ans : To have a single monochloride, there should be only one type of H-atom in the isomer of the alkane of the molecular formula C5H12. This is because, replacement of any H-atom leads to the formation of the same product. The isomer is neopentane.

Neopentane
- Qstn #4-iiThree isomeric monochlorides.Ans : To have three isomeric monochlorides, the isomer of the alkane of the molecular formula C5H12 should contain three different types of H-atoms.
Therefore, the isomer is n-pentane. It can be observed that there are three types of H atoms labelled as a, b and c in n-pentane.

- Qstn #4-iiiFour isomeric monochlorides.Ans : To have four isomeric monochlorides, the isomer of the alkane of the molecular formula C5H12 should contain four different types of H-atoms. Therefore, the isomer is 2-methylbutane. It can be observed that there are four types of H-atoms labelled as a, b, c, and d in 2-methylbutane.