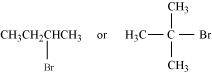NEET-XII-Chemistry
01: Haloalkanes and Haloarenes
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #6-iBromomethane, Bromoform, Chloromethane, Dibromomethane.Ans :

For alkyl halides containing the same alkyl group, the boiling point increases with an increase in the atomic mass of the halogen atom.
Since the atomic mass of Br is greater than that of Cl, the boiling point of bromomethane is higher than that of chloromethane.
Further, for alkyl halides containing the same alkyl group, the boiling point increases with an increase in the number of halides. Therefore, the boiling point of Dibromomethane is higher than that of chloromethane and bromomethane, but lower than that of bromoform.
Hence, the given set of compounds can be arranged in the order of their increasing boiling points as:
Chloromethane < Bromomethane < Dibromomethane < Bromoform.
- Qstn #6-ii1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.Ans :
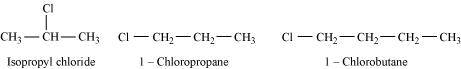
For alkyl halides containing the same halide, the boiling point increases with an increase in the size of the alkyl group. Thus, the boiling point of 1-chlorobutane is higher than that of isopropyl chloride and 1-chloropropane.
Further, the boiling point decreases with an increase in branching in the chain. Thus, the boiling point of isopropyl alcohol is lower than that of 1-chloropropane.
Hence, the given set of compounds can be arranged in the increasing order of their boiling points as:
Isopropyl chloride < 1-Chloropropane < 1-Chlorobutane
- Qstn #7Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
- Ans :

2-bromobutane is a 2° alkylhalide whereas 1-bromobutane is a 1° alkyl halide. The approaching of nucleophile is more hindered in 2-bromobutane than in 1-bromobutane. Therefore, 1-bromobutane reacts more rapidly than 2-bromobutane by an SN2 mechanism.
- Ans :
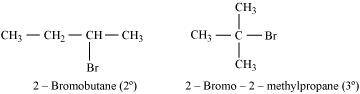
2-Bromobutane is 2° alkylhalide whereas 2-bromo-2-methylpropane is 3° alkyl halide. Therefore, greater numbers of substituents are present in 3° alkyl halide than in 2° alkyl halide to hinder the approaching nucleophile. Hence, 2-bromobutane reacts more rapidly than 2-bromo-2-methylpropane by an SN2 mechanism.
- Ans :
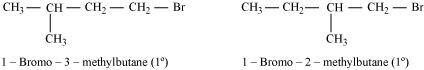
Both the alkyl halides are primary. However, the substituent -CH3 is at a greater distance to the carbon atom linked to Br in 1-bromo-3-methylbutane than in 1-bromo-2-methylbutane. Therefore, the approaching nucleophile is less hindered in case of the former than in case of the latter. Hence, the former reacts faster than the latter by SN2 mechanism.
- Ans :

SN1 reaction proceeds via the formation of carbocation. The alkyl halide (I) is 3° while (II) is 2°. Therefore, (I) forms 3° carbocation while (II) forms 2° carbocation. Greater the stability of the carbocation, faster is the rate of SN1 reaction. Since 3° carbocation is more stable than 2° carbocation. (I), i.e. 2-chloro-2-methylpropane, undergoes faster SN1 reaction than (II) i.e., 3-chloropentane.