NEET-XII-Chemistry
01: Haloalkanes and Haloarenes
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Ans :

The alkyl halide (I) is 2° while (II) is 1°. 2° carbocation is more stable than 1° carbocation. Therefore, (I), 2-chloroheptane, undergoes faster SN1 reaction than (II), 1-chlorohexane.
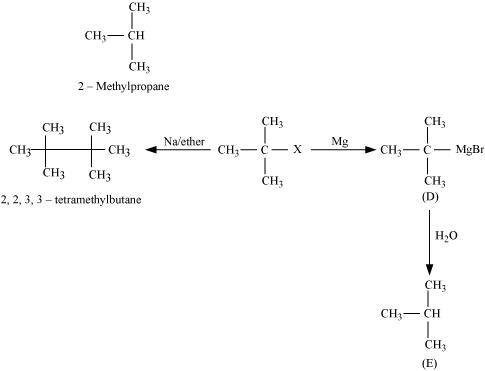
- Qstn #9Identify A, B, C, D, E, R and R1 in the following:
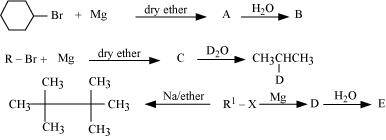
Ans :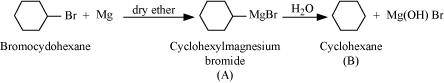
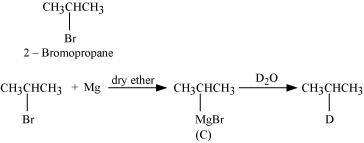
Since D of D2O gets attached to the carbon atom to which MgBr is attached, C is
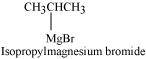
Therefore, the compound R - Br is
When an alkyl halide is treated with Na in the presence of ether, a hydrocarbon containing double the number of carbon atoms as present in the original halide is obtained as product. This is known as Wurtz reaction. Therefore, the halide, R1-X, is
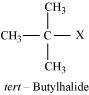
Therefore, compound D is
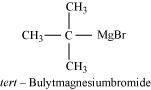
And, compound E is
- #Section : IISECTION I Page No 310:
- Qstn #1Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
- Qstn #1-i(CH3)2CHCH(Cl)CH3
() CH3CH2CH(CH3)CH(C2H5)Cl
() CH3CH2C(CH3)2CH2I
() (CH3)3CCH2CH(Br)C6H5
() CH3CH=CHC(Br)(CH3)2
() CH3CH(CH3)CH(Br)CH3
() CH3C(C2H5)2CH2Br
() CH3C(Cl)(C2H5)CH2CH3
() CH3CH=C(Cl)CH2CH(CH3)2
() p-ClC6H4CH2CH(CH3)2
() m-ClCH2C6H4CH2C(CH3)3
() o-Br-C6H4CH(CH3)CH2CH3Ans :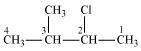
2-Chloro-3-methylbutane
(Secondary alkyl halide)
()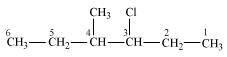
3-Chloro-4-methyhexane
(Secondary alkyl halide)
()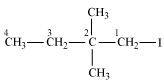
1-Iodo-2, 2 -dimethylbutane
(Primary alkyl halide)
()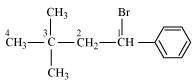
1-Bromo-3, 3-dimethyl-1-phenylbutane
(Secondary benzyl halide)
()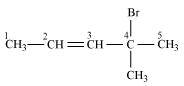
4-Bromo-4-methylpent-2-ene
(Allyl halide)
()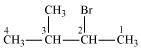
2-Bromo-3-methylbutane
(Secondary alkyl halide)
()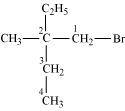
1-Bromo-2-ethyl-2-methylbutane
(Primary alkyl halide)
()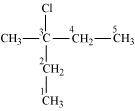
3-Chloro-3-methylpentane
(Tertiary alkyl halide)
()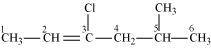
3-Chloro-5-methylhex-2-ene
(Vinyl halide)
()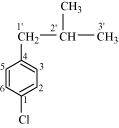
1-Chloro-4-(2-methylpropyl) benzene
(Aryl halide)
()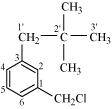
1-Chloromethyl-3-(2, 2-dimethylpropyl) benzene
(Primary benzyl halide)
()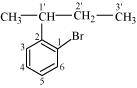
1-Bromo-2-(1-methylpropyl) benzene
(Aryl halide)
- Qstn #1-iv(CH3)3CCH2CH(Br)C6H5Ans :

1-Bromo-3, 3-dimethyl-1-phenylbutane
(Secondary benzyl halide)
- Qstn #1-xim-ClCH2C6H4CH2C(CH3)3Ans :

1-Chloromethyl-3-(2, 2-dimethylpropyl) benzene
(Primary benzyl halide)