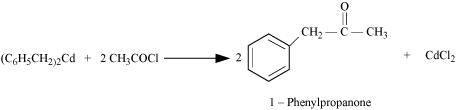
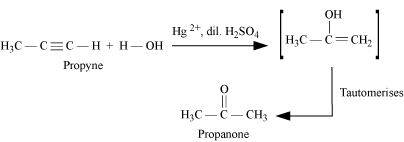
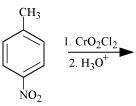
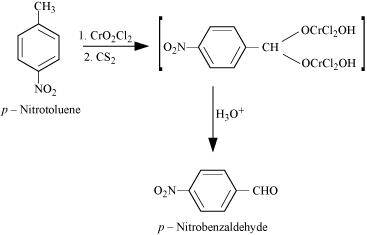
NEET-XII-Chemistry
03: Aldehydes Ketones and Carboxylic Acids
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
03-Aldehydes Ketones and Carboxylic Acids
Note: Please signup/signin free to get personalized experience.
- #3 - Aldehydes, Ketones and Carboxylic Acids
- #Section : ISECTION I Page No 353:
- Qstn #3Arrange the following compounds in increasing order of their boiling points.
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3
Ans : The molecular masses of the given compounds are in the range 44 to 46. CH3CH2OH undergoes extensive intermolecular H-bonding, resulting in the association of molecules. Therefore, it has the highest boiling point. CH3CHO is more polar than CH3OCH3 and so CH3CHO has stronger intermolecular dipole - dipole attraction than CH3OCH3⋅ CH3CH2CH3 has only weak van der Waals force. Thus, the arrangement of the given compounds in the increasing order of their boiling points is given by:
CH3CH2CH3 < CH3OCH3 < CH3CHO < CH3CH2OH