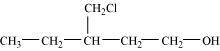NEET-XII-Chemistry
02: Alcohols, Phenols and Ethers
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
02-Alcohols, Phenols and Ethers
Note: Please signup/signin free to get personalized experience.
- #3
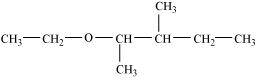
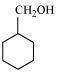
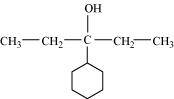
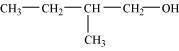
- Qstn #3-iDraw the structures of all isomeric alcohols of molecular formula C5H12O and give their IUPAC names.Ans : The structures of all isomeric alcohols of molecular formula, C5H12O are shown below:
(a)
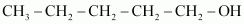
Pentan-1-ol (1°)
(b)
2-Methylbutan-1-ol (1°)
(c)
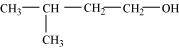
3-Methylbutan-1-ol (1°)
(d)
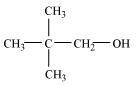
2, 2-Dimethylpropan-1-ol (1°)
(e)
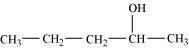
Pentan-2-ol (2°)
(f)
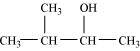
3-Methylbutan-2-ol (2°)
(g)
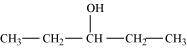
Pentan-3-ol (2°)
(h)
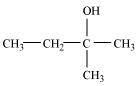
2-Methylbutan-2-ol (3°)
- Qstn #3-iiClassify the isomers of alcohols in Question 3 (i) as primary, secondary and tertiary alcohols.Ans : Primary alcohol: Pentan-1-ol; 2-Methylbutan-1-ol;
3-Methylbutan-1-ol; 2, 2-Dimethylpropan-1-ol
Secondary alcohol: Pentan-2-ol; 3-Methylbutan-2-ol;
Pentan-3-ol
Tertiary alcohol: 2-methylbutan-2-ol
- Qstn #4Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Ans : Propanol undergoes intermolecular H-bonding because of the presence of -OH group. On the other hand, butane does not
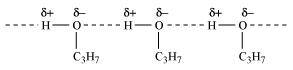
Therefore, extra energy is required to break hydrogen bonds. For this reason, propanol has a higher boiling point than hydrocarbon butane.
- Qstn #5Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Ans : Alcohols form H-bonds with water due to the presence of -OH group. However, hydrocarbons cannot form H-bonds with water.

As a result, alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses.
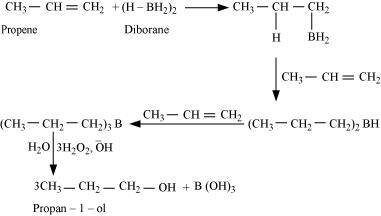
- Qstn #6What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Ans : The addition of borane followed by oxidation is known as the hydroboration-oxidation reaction. For example, propan-1-ol is produced by the hydroboration-oxidation reaction of propene. In this reaction, propene reacts with diborane (BH3)2 to form trialkyl borane as an addition product. This addition product is oxidized to alcohol by hydrogen peroxide in the presence of aqueous sodium hydroxide.
- Qstn #7Give the structures and IUPAC names of monohydric phenols of molecular formula, C7H8O.
Ans :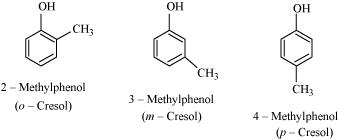
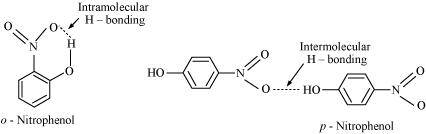
- Qstn #8While separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which will be steam volatile. Give reason.
Ans : Intramolecular H-bonding is present in o-nitrophenol. In p-nitrophenol, the molecules are strongly associated due to the presence of intermolecular bonding. Hence, o-nitrophenol is steam volatile.
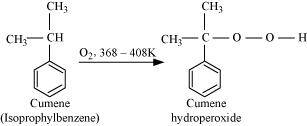
- Qstn #9Give the equations of reactions for the preparation of phenol from cumene.
Ans : To prepare phenol, cumene is first oxidized in the presence of air of cumene hydro-peroxide.
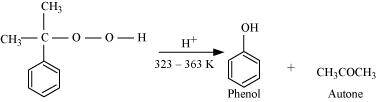
Then, cumene hydroxide is treated with dilute acid to prepare phenol and acetone as by-products.
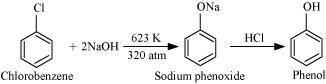
- Qstn #10Write chemical reaction for the preparation of phenol from chlorobenzene.
Ans : Chlorobenzene is fused with NaOH (at 623 K and 320 atm pressure) to produce sodium phenoxide, which gives phenol on acidification.