NEET-XII-Chemistry
07: The p-Block Elements
- #7Bond angle in
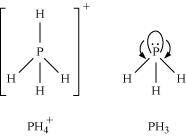
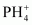 is higher than that in PH3. Why?Ans : In PH3, P is sp3 hybridized. Three orbitals are involved in bonding with three hydrogen atoms and the fourth one contains a lone pair. As lone pair-bond pair repulsion is stronger than bond pair-bond pair repulsion, the tetrahedral shape associated with sp3 bonding is changed to pyramidal. PH3 combines with a proton to form
is higher than that in PH3. Why?Ans : In PH3, P is sp3 hybridized. Three orbitals are involved in bonding with three hydrogen atoms and the fourth one contains a lone pair. As lone pair-bond pair repulsion is stronger than bond pair-bond pair repulsion, the tetrahedral shape associated with sp3 bonding is changed to pyramidal. PH3 combines with a proton to form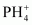 in which the lone pair is absent. Due to the absence of lone pair in
in which the lone pair is absent. Due to the absence of lone pair in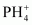 , there is no lone pair-bond pair repulsion. Hence, the bond angle in
, there is no lone pair-bond pair repulsion. Hence, the bond angle in 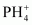 is higher than the bond angle in PH3.
is higher than the bond angle in PH3.