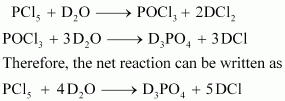NEET-XII-Chemistry
07: The p-Block Elements
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
07-The p-Block Elements
Note: Please signup/signin free to get personalized experience.
- #7 - The p-Block Elements
- #Section : ISECTION I Page No 169:
- Qstn #1Why are pentahalides more covalent than trihalides?
Ans : In pentahalides, the oxidation state is +5 and in trihalides, the oxidation state is +3. Since the metal ion with a high charge has more polarizing power, pentahalides are more covalent than trihalides.
- Qstn #2Why is BiH3 the strongest reducing agent amongst all the hydrides of
Group 15 elements?
Ans : As we move down a group, the atomic size increases and the stability of the hydrides of group 15 elements decreases. Since the stability of hydrides decreases on moving from NH3 to BiH3, the reducing character of the hydrides increases on moving from NH3 to BiH3.
- Qstn #3Why is N2 less reactive at room temperature?
Ans : The two N atoms in N2 are bonded to each other by very strong triple covalent bonds. The bond dissociation energy of this bond is very high. As a result, N2 is less reactive at room temperature.
- Qstn #4Mention the conditions required to maximise the yield of ammonia.
Ans : Ammonia is prepared using the Haber’s process. The yield of ammonia can be maximized under the following conditions:
(i) High pressure (∼ 200 atm)
(ii) A temperature of ∼700 K
(iii) Use of a catalyst such as iron oxide mixed with small amounts of K2O and Al2O3
- Qstn #5How does ammonia react with a solution of Cu2+?
Ans : NH3 acts as a Lewis base. It donates its electron pair and forms a linkage with metal ion.
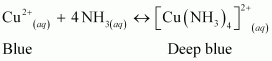
- Qstn #6What is the covalence of nitrogen in N2O5?
Ans :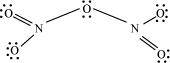
From the structure of N2O5, it is evident that the covalence of nitrogen is 4.
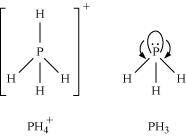
- Qstn #7Bond angle in
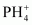 is higher than that in PH3. Why?Ans : In PH3, P is sp3 hybridized. Three orbitals are involved in bonding with three hydrogen atoms and the fourth one contains a lone pair. As lone pair-bond pair repulsion is stronger than bond pair-bond pair repulsion, the tetrahedral shape associated with sp3 bonding is changed to pyramidal. PH3 combines with a proton to form
is higher than that in PH3. Why?Ans : In PH3, P is sp3 hybridized. Three orbitals are involved in bonding with three hydrogen atoms and the fourth one contains a lone pair. As lone pair-bond pair repulsion is stronger than bond pair-bond pair repulsion, the tetrahedral shape associated with sp3 bonding is changed to pyramidal. PH3 combines with a proton to form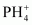 in which the lone pair is absent. Due to the absence of lone pair in
in which the lone pair is absent. Due to the absence of lone pair in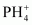 , there is no lone pair-bond pair repulsion. Hence, the bond angle in
, there is no lone pair-bond pair repulsion. Hence, the bond angle in 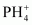 is higher than the bond angle in PH3.
is higher than the bond angle in PH3.
- Qstn #8What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2?
Ans : White phosphorous dissolves in boiling NaOH solution (in a CO2 atmosphere) to give phosphine, PH3.

- Qstn #9What happens when PCl5 is heated?
Ans : All the bonds that are present in PCl5 are not similar. It has three equatorial and two axial bonds. The equatorial bonds are stronger than the axial ones. Therefore, when PCl5 is heated strongly, it decomposes to form PCl3.

- Qstn #11What is the basicity of H3PO4?
Ans : H3PO4
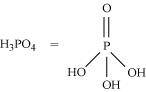
Since there are three OH groups present in H3PO4, its basicity is three i.e., it is a tribasic acid.
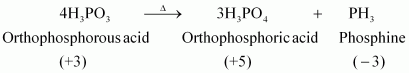
- Qstn #12What happens when H3PO3 is heated?
Ans : H3PO3, on heating, undergoes disproportionation reaction to form PH3 and H3PO4. The oxidation numbers of P in H3PO3, PH3, and H3PO4 are +3, -3, and +5 respectively. As the oxidation number of the same element is decreasing and increasing during a particular reaction, the reaction is a disproportionation reaction.
- Qstn #13List the important sources of sulphur.
Ans : Sulphur mainly exists in combined form in the earth’s crust primarily as sulphates [gypsum (CaSO4.2H2O), Epsom salt (MgSO4.7H2O), baryte (BaSO4)] and sulphides [(galena (PbS), zinc blends (ZnS), copper pyrites (CuFeS2)].