NEET-XII-Chemistry
02: Solutions
- #22At 300 K, 36 g of
glucose present in a litre of its solution has an osmotic pressure of
4.98 bar. If the osmotic pressure of the solution is 1.52 bars at the
same temperature, what would be its concentration?Ans : Here,
T = 300 K
π = 1.52 bar
R = 0.083 bar L K-1 mol-1
Applying the relation,
π = CRT
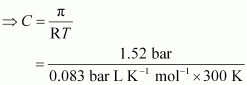
= 0.061 mol
Since the volume of the
solution is 1 L, the concentration of the solution would be 0.061 M.