NEET-XI-Chemistry
06: Hydrocarbons
- #18Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give reason for this behaviour.
Ans : Acidic character of a species is defined on the basis of ease with which it can lose its H-atoms.
The hybridization state of carbon in the given compound is:
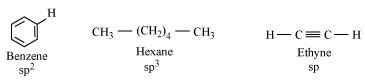
As the s-character increases, the electronegativity of carbon increases and the electrons of C-H bond pair lie closer to the carbon atom. As a result, partial positive charge of H-atom increases and H+ ions are set free.
The s-character increases in the order:
sp3 < sp2 < sp
Hence, the decreasing order of acidic behaviour is Ethyne > Benzene > Hexane.