NEET-XI-Chemistry
06: Hydrocarbons
- #3For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated:
(a) C4H8 (one double bond)
(b) C5H8 (one triple bond)
(b) C5H8 (one triple bond)Ans : null (a) The following structural isomers are possible for C4H8 with one double bond:
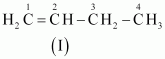
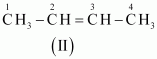
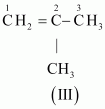
The IUPAC name of
Compound (I) is But-1-ene,
Compound (II) is But-2-ene, and
Compound (III) is 2-Methylprop-1-ene.
(b) The following structural isomers are possible for C5C8 with one triple bond:
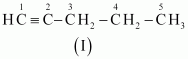
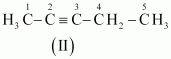
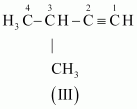
The IUPAC name of
Compound (I) is Pent-1-yne,
Compound (II) is Pent-2-yne, and
Compound (III) is 3-Methylbut-1-yne.
(b) The following structural isomers are possible for C5C8 with one triple bond:
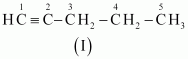
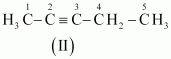
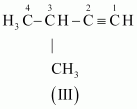
The IUPAC name of
Compound (I) is Pent-1-yne,
Compound (II) is Pent-2-yne, and
Compound (III) is 3-Methylbut-1-yne.
- #3-aC4H8 (one double bond)
(b) C5H8 (one triple bond)Ans : The following structural isomers are possible for C4H8 with one double bond:
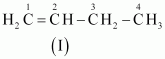
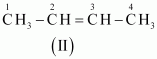
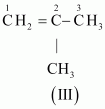
The IUPAC name of
Compound (I) is But-1-ene,
Compound (II) is But-2-ene, and
Compound (III) is 2-Methylprop-1-ene.
(b) The following structural isomers are possible for C5C8 with one triple bond:
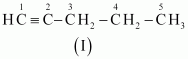
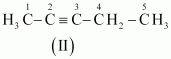
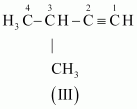
The IUPAC name of
Compound (I) is Pent-1-yne,
Compound (II) is Pent-2-yne, and
Compound (III) is 3-Methylbut-1-yne.
- #3-bC5H8 (one triple bond)Ans : The following structural isomers are possible for C5C8 with one triple bond:
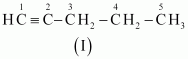
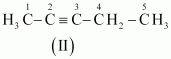
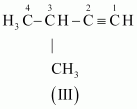
The IUPAC name of
Compound (I) is Pent-1-yne,
Compound (II) is Pent-2-yne, and
Compound (III) is 3-Methylbut-1-yne.